Iron deficiency in patients with coronary artery disease
The importance of iron as a trace element necessary for the life is beyond doubt. Iron ions are involved in oxygen transport and storage, cellular respiration in skeletal and cardiac muscles, synthesis and breakdown of proteins, lipids, carbohydrates, ribonucleic and deoxyribonucleic acids [1-3].
A number of studies have demonstrated the negative impact of iron deficiency (ID) on the prognosis and course of heart failure (HF). It has been shown that the correction of ID with parenteral iron therapy improves the prognosis, quality of life and functional capacity of patients with ID and HF, even in the absence of anemia [4-8]. One of the most common causes of HF is coronary artery disease (CAD). The prevalence of patients with CAD in the above studies was 39,4-65%, while the proportion of patients with myocardial infarction (MI) reached 60% [4-8]. Despite the fact that ID is widespread in patients with CAD [9-11], the effect of ID on the course of CAD, in particular in patients with MI, has not been fully studied, but it can be assumed that ID contributes to CAD deterioration and negatively affects on the prognosis of these patients. However, further research is needed to explore this issue. The purpose of this literature review is to analyze published data on the effect of ID on heart function, quality of life, and prognosis in patients with CAD.
Search and selection of publications
Search and selection of publications on studies related to ID and CAD was carried out in two following databases: the Cochrane Database of Systematic Reviews (http://www.thecochranelibrary.com) and Medline database (http://www. ncbi.nlm.nih.gov/pubmed), as well as with Google Scholar search engine using search queries, keywords (including MeSH) and logical operators. English and Russian has been set as the language limit. The last search was carried out on May 22, 2022. There were following keywords in the PubMed database: ((Acute coronary syndrome) OR (myocardial infarction) OR (ischemic heart disease) AND (iron deficiency) OR (ferritin)). In the Google Scholar, search queries were performed for the following keywords: ID, CAD, MI, acute coronary syndrome (ACS), ferritin, prognosis, left ventricular (LV) remodeling. A total of 359 articles were analyzed, on the basis of which a list of 29 representative publications was formed (Table 1). Four main directions of the research question were identified: pathophysiological aspects of ID effect of on the heart in CAD, the prognostic value of ID in CAD, the effect of ID on the quality of life and functional ability of patients with CAD, the effect of ID and its correction on systolic function and LV remodeling.
Pathophysiological aspects of the effect of ID on the heart
From a variety of sources describing the role of iron ions in the functioning of cardiomyocytes, we have selected 12 works directly related to the pathophysiological effect of a decrease in iron levels on the function and structure of cardiomyocytes under hypoxic conditions. The myocardium needs a lot of energy, produced mainly in the mitochondria. Iron ions are necessary for the normal functioning of mitochondria. Almost a third of all iron in cardiomyocytes is distributed in mitochondria [12]. Iron metabolism and the effect of iron overload on the structure and function of cardiomyocytes are described in most detail in a review by Ravingerov? T, et al. (2020) [13].
We did not find studies indicating a positive or neutral effect of systemic ID on cardiomyocytes. Chang H-Ch, et al. (2016) on mice models with myocardial ischemia in vivo, using the overexpression of the ABCB8 gene in the cardiomyocyte and an iron chelator (2,2’-bipyridine), reduced the level of mitochondrial iron below the initial level. Reduced mitochondrial iron has been shown to protect the myocardium from ischemic injury by reducing the area of necrosis, as assessed by in vivo echocardiography and in vitro flow cytometry [14].
The study by Hoes M, et al. (2018) demonstrated a decrease of iron in heart cells with systemic ID, even in the absence of anemia. The inability of cardiomyocytes in the condition of ID to transport and use oxygen in sufficient volume was shown, which leads to mitochondrial dysfunction, a decrease in the number of mitochondria, a change in their structure, a decrease in the synthesis of adenosine triphosphate, and a switch from fatty acid oxidation to anaerobic glycolysis. ID in the cardiomyocyte leads to severe endoplasmic reticulum damage with the formation of large perinuclear vacuoles. These changes were observed already after 4 days of cardiomyocytes being in conditions of low iron content. When replenishing iron stores, the synthesis of adenosine triphosphate, mitochondrial respiration, and the structure of the endoplasmic reticulum were restored. Thus, it was shown that the cellular effects of ID are reversible [15].
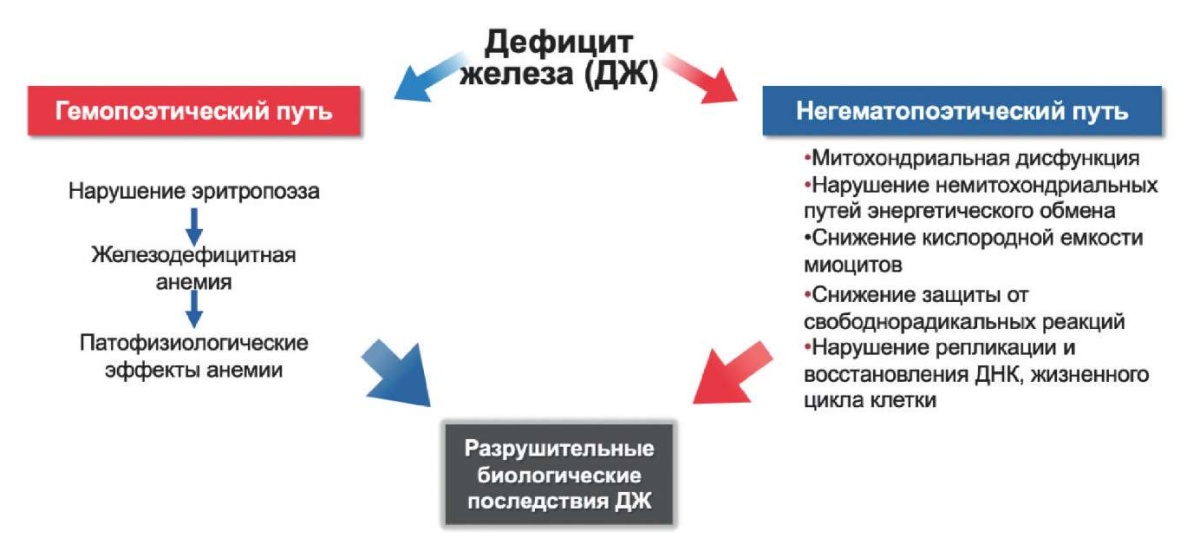
A decrease in the concentration of intracellular iron leads to an acceleration of apoptosis and a decrease in the viability of cardiomyocytes [16]. Two studies by Dziegala M, et al. (2016) and Isoda M, et al. (2010) in rat models showed that hypoxia impairs the viability of cardiomyocytes under ID conditions to a greater extent than under iron excess. Under conditions of acute ischemia and myocardial necrosis, the level of iron in the cardiomyocyte increases as a result of the cell protective reaction [17][18]. In patients with ID, such a sharp increase in cardiac iron is not observed, and therefore, in MI under conditions of ID, cardiomyocytes are subject to severe atrophy and apoptosis [19]. In addition, macrophages accumulating in the myocardium during MI absorb iron and, as a result, shift their immunological profile towards an anti-inflammatory phenotype [20]. In this connection, it can be assumed that iron has a protective immunomodulatory effect on macrophages, leading to a decrease in the area of necrosis and beneficial global LV remodeling in the case of MI [21] (Figure 1).
Along with changes in the cell structure and the functioning of organelles, myocardial contractility also decreases. In vitro experiments found that under conditions of low iron content, the area and rate of cardiomyocyte contraction is reduced by 2 times. After the addition of transferrin-bound iron, the contractile function was completely restored, but the relaxation of cardiomyocytes was only partially normalized. These data suggest that systolic heart function may improve with iron supplementation, while diastolic function is more permanently impaired. Thus, low levels of intracellular iron lead to a decrease in systolic function and a persistent impairment of diastolic function, which has been demonstrated in vitro and confirmed in clinical practice [22][23].
Prognostic value of ID in CAD
Despite the high prevalence of ID in patients with CAD, the effect of ID on this group of patients has not been studied enough. This may be due to the fact that the diagnosis of ID is not included in the standard list of examinations of patients without anemia, especially in the acute period. We analyzed 11 studies demonstrating the effect of ID on the development of CAD and the prognosis of patients with diagnosed CAD, in particular with myocardial infarction. According to the presented studies, the prevalence of ID in patients with CAD varies within 20-60% [9][11].
Data from two previously published studies have shown an association between elevated ferritin levels and the risk of MI. When studying the Finnish population in a study by Salonen JT, et al. (1992), including 1931 patients with CAD aged 42-60 years, showed an association between elevated plasma ferritin levels and an increased risk of MI. The risk of myocardial infarction in the group of patients with a ferritin level >200 mcg/l was 2 times higher than in the group with a low level of ferritin (hazard ratio (HR) 2,2, 95% confidence interval (CI), 1,2-4,0, p<0,01) [24]. Haidari M, et al. (2001) found an association between elevated ferritin levels and the risk of premature coronary artery stenosis (HR, 1,62, 95% CI, 1,12-2,42, p<0,01). When included in the study, patients were not diagnosed with an infectious and inflammatory disease, and the level of C-reactive protein was equally normal in both groups. However, it should be noted that the results were obtained only in the group of men and did not take into account serum iron and transferrin saturation [25].
The study by Mohammadifard N, et al. (2017) showed that regular blood donation reduces the risk of ischemic events in donors. However, this fact may be due to the fact that blood donors lead a healthier lifestyle [26].
At the same time, studies of the last decade, on the contrary, indicate a negative effect of ID on the development of CAD. Thus, two meta-analyses involving >290 thousand people revealed an inverse relationship between the amount of iron in the body and the development of CAD and cardiovascular events. Normal plasma iron levels were associated with a 20% reduction in the risk of CAD and a 15% reduction in the risk of cardiovascular events and mortality compared with ID (RR, 0,80, 95% CI, 0,73-0,87 and RR 0,85, 95% CI, 0,73-0,99, respectively) [27][28].
ID has a significant impact on the prognosis of patients with CAD. We analyzed 5 studies evaluating the effect of ID on the risk of all-cause and cardiovascular mortality, the incidence of ACS, in particular non-fatal MI, both during hospitalization and during longer follow-up [9][11][29-31]. In the course of monitoring patients with established diagnoses of CAD (according coronary angiography in 2162 people) for 10 years, a relationship between ID and mortality was established. Patients with ID, regardless of anemia, were at higher risk of cardiovascular and all-cause mortality (HR, 1,27, 95% CI, 1,02-1,58) [29].
A fairly common form of CAD is MI; therefore, the influence of ID on CAD are often considered within the framework of MI. According to Cosentino N, et al. (2019), the prevalence of ID in patients with MI reaches 56% [11]. ID increased the risk of death over 4,7 years of follow-up in patients with MI by 54% (HR, 1,54, CI, 1,03-2,30, p=0,035) [30]. According to Zeller T, et al. (2018), which included follow-up of 836 patients for 4 years after hospitalization for ACS, ID influenced the prognosis of patients with MI, increasing the risk of non-fatal MI and cardiovascular death by 73% (p=0,04) [9].
ID also affects the risk of death and cardiovascular events in patients with MI during hospitalization. According to Fujinaga H, et al. (2013) patients with ID were more likely to die during hospitalization for MI compared with patients with normal iron levels (6,5% vs 1,6%, p=0,03; HR not calculated) [31]. At the same time, according to Cosentino N, et al. (2019) in patients with ID, there was a twofold reduction in the risk of unfavorable outcomes during hospitalization for MI (HR, 0,50, CI 0,27-0,93) [11] (Table 2, Figure 2). Due to the fact that the follow-up duration, endpoints and criteria for ID in the presented studies differed, conducting a metaanalysis was not possible. The summarized data are presented in Table 2.
Influence of ID on the quality of life and functional ability. In an earlier study by Mero?o O, et al. (2017) in patients with ID, authors recorded a shorter distance in the 6-minute walk test (277 vs 423 m, p=0,009), worse results in the treadmill test (HR, 2,9, 95% CI, 1,1-7,6, p=0,023) and a pronounced decrease in quality of life 30 days after ACS, compared with patients without ID (HR, 1,9, 95% CI, 1,1-3,3, p<0,001) [10].
Influence of ID and its correction on systolic function and LV remodeling. The 4 studies presented below testify to the negative effect of ID on cardiac structure in patients with CAD.
According to Paeres AE, et al. (2018) patients with MI and ferritin levels <100 ng/ml 30 days after hospitalization had a lower LV ejection fraction compared with patients with ferretin levels >100 ng/ml (51,6% and 55,5%, respectively), p=0,04) [32]. According to Huang CH, et al. (2019) serum iron levels were lower in patients who did not experience recovery of LV systolic function 6 months after percutaneous coronary intervention due to MI (52,7 mg/dl vs 80,8 mg/dl, p=0,016) [23]. Inserte J, et al. (2021) studied the effect of ID on LV myocardial remodeling in 141 patients with acute MI. In patients with ID, according to magnetic resonance imaging, a large area of necrosis was initially observed (22,8% vs 16,8% of the LV mass, p=0,002). After 6 months, LV enlargement and its remodeling were observed in 37,8% of patients with ID and only in 14,1% of patients with normal iron levels (p=0,004). The results obtained were confirmed by the authors in a model of MI in rats [33]. However, a longer-term effect of ID on LV ejection fraction has not been reported in patients with MI.
We found only 4 studies that were aimed at studying the correction of ID in CAD. According to Belousova N.S. et al. (2011), the use of iron preparations against the background of basic therapy for CAD in ID affected myocardial remodeling in patients after myocardial infarction, contributing to a significant decrease in the LV mass index (103,4±18,4 g/m2 before the use of iron preparations and 101,5±19,2 g/m2 after, p<0,0001) and an increase in the LV ejection fraction (60,8±5,4% and 67,5±5,2%, p<0,0001) [34].
In a study by Paterec A, et al. (2021) intravenous administration of iron carboxymaltose (ICM) 30 min after MI to rats with normal iron status had no effect on LV systolic function and its remodeling, on the risk of arrhythmias and death after MI, and turned out to be absolutely safe. The authors note that all rats had the same normal iron status and the effect of ICM in the presence of ID requires further study [35].
In the study by Wischmann P, et al. (2021), ICM was administered to mice with iron deficiency anemia 1 or 24 hours after acute myocardial injury. It was found that the introduction of ICM 24 hours after MI significantly reduced LV remodeling, which is expressed in a decrease in end-systolic volume, and also led to a decrease in myocardium damaged area. These positive effects were not observed in the placebo group and in the group in which ICM was administered 1 hour after MI. Thus, the authors conclude not only about the need for the introduction of ICM in myocardial infarction in conditions of ID, but also the importance of observing time intervals [36].
In the previously mentioned work by Inserte J, et al. (2021) the use of an iron-rich diet or the administration of iron sucrose to rats with ID reduced the severity of oxidative stress and led to a decrease in necrosis area [33].
Table 1
Search and selection of publications


Figure 1. Pathophysiological mechanisms of ID influence of on the heart. Adapted from [7].
Abbreviations: ID — iron deficiency, DNA — deoxyribonucleic acid.
Table 2
Influence of ID on the risk of non-fatal MI, all-cause and cardiovascular mortality in patients with MI

Abbreviations: ID — iron deficiency, CI — confidence interval, MI — myocardial infarction, ACS — acute coronary syndrome, HR — hazard ratio.

Figure 2. Influence of ID on the risk of non-fatal MI, all-cause and cardiovascular mortality in patients with MI.
Abbreviations: CI — confidence interval, HR — hazard ratio.
Conclusion
A systematic analysis of publications concerning the effect of ID on patients with CAD was carried out. There are 4 following main areas of research:
1. Pathophysiological aspects of ID effect on the heart in conditions of CAD;
2. Prognostic value of ID in CAD;
3. Influence of ID on the quality of life and functional ability of patients with CAD;
4. Influence of ID and its correction on systolic function and LV remodeling.
Most of the studies conducted to date indicate the negative impact of ID on the structure and function of the heart, quality of life and prognosis in patients with CAD. ID correction reduced the amount of damage and necrosis during ischemia and MI, improved myocardial systolic function and exercise tolerance after MI. However, the available data are scarce, and the problem of ID in patients with various CAD types requires further study.
Relationships and Activities: none.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Khastieva D.R., Khasanov N.R. Iron deficiency in patients with coronary artery disease. Russian Journal of Cardiology. 2022;27(4S):4962. https://doi.org/10.15829/1560-4071-2022-4962
Copy