Role of heart rate variability and regulatory-adaptive status index in predicting the heart transplant rejection
Heart transplantation is the only radical treatment for patients with severe heart failure [1]. Advances in the immunosuppressive therapy and the surgical technique of transplantation, and lifelong follow-up at transplant centers have improved the survival rate and quality of life of recipients, but the problem of cardiac allograft rejection remains extremely urgent. The procedure of endomyocardial biopsy (EMB), performed annually and according to indications, is the main method for diagnosing a rejection [1]. Invasiveness, high cost, risk of complications of this manipulation served as an incentive to search for safe, non-invasive and simple methods for predicting a rejection episodes, which would allow considering EMB after the early postoperative period. Currently, Russian and foreign colleagues offer both laboratory and functional methods for predicting allograft rejection. The most modern are the study of plasma miRNA-101 and miRNA-27, as well as speckle-tracking echocardiography [2][3]. Our study assessed the diagnostic value of methods aimed at diagnosing graft reinnervation in predicting allograft rejection.
Material and methods
The study included 70 patients after orthotopic heart transplantation using a modified bicaval technique performed in the period of 2012-2015. Most of the recipients were men (n=59; 84,29%). The mean age of the study group was 50,24±10 years. The indications for heart transplantation were ischemic cardiomyopathy in 30 (42,86%), dilated cardiomyopathy in 33 (47,14%), myocarditis in 4 (5,71%), and valvular heart disease in 3 (4,29%) patients. The follow-up period was 36±1 months from the surgery date. Drug therapy after transplantation included the following drug groups: calcineurin inhibitors (tacrolimus at a target concentration of 10-15 ng/ml for 1 year, then 5-10 ng/ml), mycophenolic acid, glucocorticoids (prednisolone 1 mg/kg with a gradual dose reduction and subsequent withdrawal 1 year after transplantation), acetylsalicylic acid, statins, angiotensinconverting enzyme inhibitors. The study included patients with sinus rhythm, without an artificial pacemaker, and not taking heart rate lowering drugs. Twelve, 24 and 36 months after surgery, all the subjects, along with the standard examination after heart transplantation, underwent 24-hour electrocardiographic (ECG) monitoring, as well as cardiorespiratory synchronization (CRS).
24-hour ECG monitoring was carried out for 24 hours using the Cardiotekhnika-07 system (Inkart, St. Petersburg). In order to rule out possible arrhythmias and distortion of heart rate variability (HRV) due to invasive procedures, the study was performed 48 hours after EMB and coronary angiography. The results were analyzed using the KT Result 2 (Expert) program (Inkart, St. Petersburg). The average 24-hour heart rate (HR), QRS complex, heart rhythms and arrhythmias, and standard HRV parameters were analyzed sequentially.
To perform the CRS, we used the VNS-Micro device (Neurosoft, Russia) and the computer program “System for assessing cardiorespiratory synchronization in humans” (Pokrovsky V.M. et al., 2009) [4]. Simultaneously, ECG and spirography was performed. Next, photic stimulation was carried out, followed by automatic registration of synchronization between the given respiratory rhythm and heart rate. A few minutes after the end, necessary to restore heart rate and respiration at the initial level, the tests were repeated with a subsequent 5% increase in the frequency of expiratory photic. The frequency range of cardiorespiratory synchronization was determined by registering the maximum and minimum limits of synchronization onset. The Index of Regulatory Adaptive Status (IRAS) was calculated using the following equation: IRAS=SR/Dmin*100, where SR is the synchronization range, Dmin — duration of synchronization development at the minimum boundary of range. Regulatory-adaptive capabilities were assessed depending on the IRAS: IRAS >100 — high, 50-99 — good, 24-49 — satisfactory, 9-23 — low, <9 — unsatisfactory [4].
EMB was performed annually according to the standard method. In parallel with the pathological study, an immunohistochemistry was carried out in order to detect antibody-mediated rejection. The results were interpreted in accordance with the cellular and humoral rejection classification of the International Society for Heart and Lung Transplantation (ISHLT).
Statistical processing was carried out using the Statistica 10 program (StatSoft Inc., version 10.0.228.8, Oklahoma, USA). The following methods were used: frequency analysis of adverse outcomes; statistical comparison of the proportions of adverse outcome frequency in different observation periods (z-test for proportions); statistical comparisons of mean HRV and SDS with and without rejection episode in different time periods (Mann-Whitney test with a significance level of p<0,05), median, interquartile range (Me (25%-75%)); ROC-analysis of HRV and CDS values in predicting rejection episodes.
The study was performed in accordance with Good Clinical Practice and Declaration of Helsinki principles. Written informed consent was obtained from all participants prior to enrollment. The local ethics committee approved this study.
Results
According to the EMB results, 1 year after transplantation, 23 (33%) recipients had verified cellular rejection as follows: 1R — 16, 2R — 6, 3R — 1 patient. Twenty-four months after surgery, there were 23 (34,8%) rejections as follows: cellular rejection 1R — 19, 2R — 2, patients, while 2 patients had humoral rejection AMR 2. Tree years after heart transplantation, 19 (29,6%) patients developed cellular rejection, among whom 15 patients had 1R and 3 — 2R. During the follow-up period, there were 6 deaths, among which 4 were between the 1st and 2nd year, and 2 between the 2nd and 3rd year.
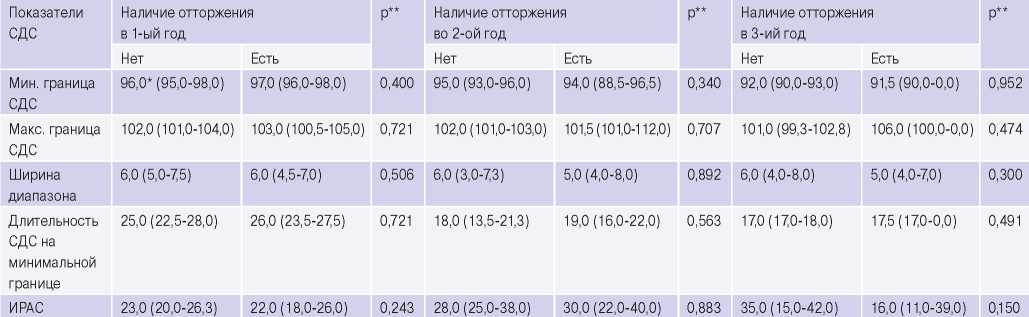
Statistical comparisons of mean HRV and CDS values show that after 1 year, there were no significant differences in all HRV and SDS indicators between the samples with and without a rejection episodes (Tables 1, 2). No significant CDS differences were found in the subsequent follow-up years (p>0,3 in the 2nd and 3rd years), which indicates the absence of significant CDS alterations in rejection (Table 2).
As for HRV in the second follow-up year, significant differences between groups were in average 24-hour heart rate (p=0,038), the high-frequency (HF) component (p=0,010) and the root mean square successive difference (rMSSD) of RR (p=0,040) (Table 1). After 36 months, differences were already recorded for many HRV parameters as follows: heart rate, HF, rMSSD, low-frequency (LF) component (p<0,001); the standard deviation of the normal-tonormal RR intervals (SDNN) (p<0,05); very low frequency component (VLF) (p<0,01) (Table 1). According to the medians of studied parameters, an increase/decrease in significant HRV indicators can be assessed.
According to ROC analysis, 2 years after surgery, HF component had the highest predictive value (AUC=0,693 at p=0,005) (Table 3). Compared to heart rate and rMSSD, this parameter has approximately equal sensitivity (Se=73,9) and specificity (Sp=72,1). With HF component ?12 ms2, there is a 73,9% chance that a patient will be assigned to the rejection group. The positive predictive value of HF component is 58,6%, while the negative predictive value — 83,8%. Thus, this indicator may be the most effective in predicting the absence of graft rejection.
Three years after the operation, all the analyzed HRV parameters were informative for diagnosing the rejection (Table 4). According to the obtained data, at the 3rd follow-up year, heart rate had the highest predictive value (AUC=0,873 at p<0,001). This parameter has high sensitivity (Se=80,0) and specificity (Sp=90,0). With a heart rate >92, there is an 80% chance that the patient will have a rejection. High positive (80,0) and negative (90,0) predictive values determine heart rate 3 years after surgery as the most valuable predictor of rejection. It should be noted that among the parameters characterizing the cardiac autonomic regulation, parasympathetic reinnervation of both frequency and time domains have the highest predictive value: HF (AUC=0,814 at p<0,001) and rMSSD (AUC=0,882 at p<0,001). ROC analysis revealed that the SDNN has lower specificity and sensitivity compared to the heart rate (75% vs 80%, 56,8% vs 90,9%). The LF and VLF components are not very sensitive, which makes them less effective for predicting rejection in heart recipients in the 3rd year after surgery.
Table 1
Comparison of mean HRV parameters with/without a rejection episode

Note: * — median and interquartile range (Me (25%-75%)); ** — a non-parametric Mann-Whitney test was used; the significance was p<0,05.
Abbreviations: HR — heart rate, HF — high-frequency component, LF — low-frequency component, rMSSD — root mean square successive difference of RR, SDANN — standard deviation of 5-minute normal-to-normal RR intervals, SDNN — standard deviation of normal-to-normal RR intervals for the entire period under consideration, VLF — very low frequency component.
Table 2
Comparison of mean CRS parameters with/without a rejection episode

Note* — median and interquartile range (Me (25%-75%)); ** — a non-parametric Mann-Whitney test was used; the significance was p<0,05.
Abbreviations: IRAS — index of regulatory-adaptive status, CRS — cardiorespiratory synchronization.
Table 3
Analysis of HRV parameters for diagnosing a rejection episode in the 2nd follow-up year (ROC analysis results)

Note: HRV — heart rate variability, CI — confidence interval, HR — heart rate, АUC±S.E. — area under the ROC curve, HF — highfrequency component, rMSSD — root mean square successive difference of RR intervals, Se — sensitivity, Sp — specificity, +PV — positive predictive value, -PV — negative predictive value.
Table 4
Analysis of HRV parameters for diagnosing a rejection episode in the 3rd follow-up year (ROC analysis results)

Note: HRV — heart rate variability, CI — confidence interval, HR — heart rate, АUC±S.E. — area under the ROC curve, HF — highfrequency component, LF — low-frequency component, rMSSD — root mean square successive difference of RR intervals, SDANN — standard deviation of 5-minute normal-to-normal RR intervals, SDNN — standard deviation of normal-to-normal RR intervals for the entire period under consideration, Se — sensitivity, Sp — specificity, VLF — very low frequency component, +PV — positive predictive value, -PV — negative predictive value.
Discussion
The transplanted heart is a patent example of a complete autonomic block due to surgical denervation. This specifies the peculiarities of transplant physiology: high heart rate, absence of circadian cycle and HRV, slow heart rate increase in response to physical activity and slow recovery, no change in heart rate in response to vagal maneuvers [5]. However, over time after transplantation, there are signs of partial allograft reinnervation, which is demonstrated by the experimental and clinical studies [6][7]. The most accessible, safe and, at the same time, informative functional method for verifying reinnervation is the 24-hour ECG.
Heart rate reflects the influence of neural and humoral factors on the sinoatrial node, and the frequency and time HRV domains — the degree of parasympathetic and sympathetic influence on its function. CRS is a technique for synchronizing respiration and heart rate by means of a given frequency of voluntary breathing, usually exceeding the initial heart rate (Pokrovsky V.M., 2003). CRS includes integral parameters, since a number of processes in the central and autonomic nervous system, respiratory system and in the heart itself are involved in CRS. The synchronization is based on the transformation of a given visual signal into a command of control breathing with a frequency corresponding to photic stimulation, the introduction of respiratory and cardiovascular center interactions and the synchronization of their rhythms, followed by sending impulses along the vagus nerve, interaction of signals with the heart’s rhythmogenic structures and reproduction by the heart of rate set by breathing [4]. Based on the above mechanism of CRS development, it becomes clear that the very fact of a positive CRS reflects the heart transplant reinnervation, and the IRAS value reflects its severity. These research methods were chosen by us in order to predict the rejection from the standpoint of transplant physiology. According to the results obtained, 1 year after the operation, none of the HRV and CRS parameters were associated with the rejection, which is explained by the short period after operation and the high heart rate rigidity. Extremely low HRV values are due to the predominance of hormonal and intracardiac regulation systems. Foreign studies showed that signs of sympathetic reinnervation are observed earlier (from 5-6 months to 1 year after surgery), while parasympathetic reinnervation requires a longer postoperative period and is recorded in the second year after surgery [8-10]. According to the longest study by Beckers F, et al., positive HRV dynamics was noted 2 years after surgery, and an increase in LF component power and, accordingly, the presence of sympathetic reinnervation — after 4 years, but LF values are lower, than in healthy people [11]. The difference in the timing and level of this indicator among heart recipients indicates that the sympathetic reinnervation is heterogeneous.
A recent review reported no relationship between allograft reinnervation and postoperative outcome [12]. However, in the described study by Bengel F, et al., only sympathetic reinnervation and its relationship with rejection were assessed. In our work, 2 years after transplantation, ROC analysis established diagnostic value for average 24-hour heart rate, HF component and rMSSD. The results obtained 3 years after the operation demonstrate a good predictive value of the average 24-hour heart rate, while the sympathetic and parasympathetic reinnervation have a lower predictive value. Taking into account the topographic and temporal heterogeneity of sympathetic and parasympathetic reinnervation, it can be concluded that by the third year after transplantation, there is a balance between the autonomic nervous regulation, their influence on the average 24-hour heart rate increases. This leads to conditions for heart rate regulation close to the physiology of a non-transplanted heart.
The prognostic relationship we expected between IRAS and rejection development during the threeyear follow-up period was not revealed. This can be explained by the insufficient follow-up period. We note some gradual increase in the synchronization range width over time. However, IRAS has a value that is regarded as satisfactory even in the third year after the operation.
Study limitations. Our study has some limitations. The relatively short follow-up period after surgery does not allow us to assess the diagnostic value of HRV and CRS in predicting rejection crises in the long-term period after transplantation, as well as their possible prognostic role in relation to allograft vasculopathy and death. Visualizing sympathetic reinnervation by positron emission tomography or myocardial scintigraphy would show a more complete picture of the autonomic allograft regulation. However, due to technical difficulties, these imaging methods was not included in the study protocol.
Conclusion
Our results make it possible to consider the HRV examination as a non-invasive, safe and easily reproducible technique for assessing the rejection risk in recipients 2 and 3 years after surgery, and the study of CRS for verifying cardiac allograft reinnervation and assessing the regulatory and adaptive capabilities of the recipient.
Relationships and Activities: none.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Lepshokova M.K., Kosmacheva E.D. Role of heart rate variability and regulatory-adaptive status index in predicting the heart transplant rejection. Russian Journal of Cardiology. 2021;26(4S):4698. https://doi.org/10.15829/1560-4071-2021-4698
Copy