Myocardial remodeling and fibroblast growth factor in patients with resistant hypertension
Hypertension (HTN) is still the leading cause of cardiovascular deaths [1]. A special place is occupied by resistant hypertension, in which blood pressure (BP) remains >140/90 mm Hg, despite the simultaneous use of 3 antihypertensive drugs, including a renin-angiotensin-aldosterone system (RAAS) inhibitor, a calcium channel antagonist and a diuretic in maximum tolerated doses [2].
Resistant hypertension is associated with more pronounced target organ damage, including left ventricular hypertrophy (LVH) [3]. According to a meta-analysis of 11 studies involving 3325 patients with resistant HTN, the prevalence of LVH varies from 55 to 75% [4], while in the population of all hypertensive patients, the prevalence of LVH ranges from 36 to 41% (in the general population ~15-20%) [5]. It has been repeatedly demonstrated that LVH is an independent predictor of cardiovascular events (CVEs) in individuals with HTN [6] and in the general population [7]. Currently, the main causes leading to the development and progression of LVH in hypertensive patients are recognized as poor BP control, age, sex, obesity, hyperactivity of the sympathetic nervous system and RAAS, low physical activity, and concomitant heart diseases [8].
In addition, the search for new pathogenetic ways for LVH development continues in order to develop modern therapeutic approaches aimed at reducing cardiovascular morbidity and mortality. In recent years, special attention has been drawn to fibroblast growth factor-23 (FGF23), a phosphaturic protein involved in the regulation of mineral metabolism, vascular calcification and being an independent risk factor for CVEs. The level of FGF23 increases with the progression of chronic kidney disease, reaching maximum values in individuals with end-stage renal disease [9]. A less pronounced increase in the concentration of FGF23 is observed with age, even in individuals with preserved or slightly reduced renal function [10]. According to experimental data, FGF23 is a direct mediator of LVH development not only in a cohort of patients with chronic kidney disease [11], but also in the general population [10]. An association between the progression of LVH and the serum level of FGF23 was also demonstrated in a model of hypertensive rats [12]. FGF23 causes pathological hypertrophy of isolated cardiomyocytes through binding to the FGFR4 receptor and activation of the calcineurin-NFAT pathway [13]. In addition to triggering prohypertrophic gene programs, FGF23 can induce LVH by increasing the expression of profibrotic factors and inflammatory cytokines in cardiomyocytes [14]. FGF23 is able to influence the renal expression of angiotensinconverting enzyme-2, thereby increasing the activity of the RAAS [15]. At the same time, in most published studies, the severity of myocardial hypertrophy depended on the plasma FGF23 concentration [10-14]. However, in some studies, the degree of left ventricular (LV) remodeling was associated with the duration of FGF23 exposure to the myocardium, regardless of its level [16]. It is well known that modern antihypertensive therapy can affect the regression of LVH and the reduction of cardiovascular events and mortality, regardless of the achievement of the target blood pressure level, including in persons with resistant HTN [17].
We hypothesized that in this cohort of patients, myocardial remodeling, in addition to generally recognized factors such as blood pressure levels, may be influenced by other factors, for example, those responsible for calcification and vascular stiffness. In the available literature, there are no studies on the relationship between LVH and FGF23 in patients with resistant HTN. In this regard, the aim of our work was to study the prevalence and severity of LVH and its relationship with fibroblast growth factor in patients with resistant HTN, depending on the effectiveness of antihypertensive therapy.
Material and methods
An open-label cross-sectional comparative study was carried out on the basis of the Rostov State Medical University and the “Health” Clinical and Diagnostic Center in Rostov-on-Don in the period from 2017 to 2021. The study protocol was approved by the local ethics committee of Rostov State Medical University. At the screening stage, a retrospective analysis of 4874 outpatient records of patients aged 18 to 70 years with a diagnosis of essential (primary) hypertension was performed, as a result of which a group of patients requiring 3 or more antihypertensive drugs was identified. Excluding patients with symptomatic HTN, patients with a decrease in glomerular filtration rate (GFR) estimated by the CKD-EPI <60 ml/min/1,73 m2 and microalbuminuria (MAU) >30 mg/day, persons with severe endocrine disorders (diabetes, thyroid dysfunction, parathyroid dysfunction, vitamin D deficiency), coronary artery disease, hemodynamically significant atherosclerosis of peripheral arteries, class III-IV heart failure, arrhythmias, as well as persons with concomitant therapy that can affect BP levels (oral contraceptives, sympathomimetics, non-steroidal antiinflammatory drugs) and FGF23 levels (vitamin D, calcimimetics, phosphate binders), 151 people with probable resistant HTN were selected, who then underwent pharmacotherapy optimization for at least 6 months. Treatment adherence was assessed using the Brief Medication Questionnaire (BMQ) and monitoring the number of pills taken. The effectiveness of therapy was monitored by home BP measurement. As a result, the study included 92 patients diagnosed with resistant HTN, established on the basis of the Russian Society of Cardiology (2020) guidelines [2], who did not reach the target BP level, despite the simultaneous use of 3 antihypertensive drugs of different classes, including a RAAS blocker, a calcium channel blocker, and diuretic at maximum tolerated doses or achieved target BP while taking ?4 antihypertensive drugs.
In all patients, the following parameters were determined: serum creatinine content with the calculation of GFR using the CKD-EPI equation, potassium, sodium, phosphorus, free and ionized calcium, uric acid, glucose, aspartate aminotransferase, alanine aminotransferase, lipid spectrum, as well as the level of 24-hour MAU. The serum level of FGF23 was determined by enzyme immunoassay using the Human FGF-23 ELISA Kit. The measurement range was 0,1-20 pmol/l, sensitivity — 0,08 pmol/l.
We performed twenty four-hour ambulatory BP monitoring (ABPM) using the Schiller BR-102 plus system (Sweden) according to the generally accepted standard method, as well as echocardiography (Toshiba500, Japan) in 3 main modes. LV diastolic function was determined by the parameters of transmitral blood flow: peak early left ventricular filling (peak E), peak late left ventricular filling (peak A) and their ratio. LV mass (LVM) was determined by the formula of the American Society of Echocardiography (ASE), LVM index (LVMI). LVH was diagnosed with LVMI >115 g/m2 in men and >95 g/m2 in women [18]. LV relative wall thickness (RWT) was calculated as the ratio of the sum of interventricular septum and LV posterior wall thickness to LV end-diastolic dimension. The type of LV geometry was determined according to the classification of A. Ganau (1992): normal LV geometry with LVMI <125 g/m2 and RWT <0,42; concentric LV remodeling with LVMI <125 g/m2 and RWT >0,42; concentric LVH with LVMI >125 g/m2 and RWT >0,42; eccentric LVH with LVMI >125 g/m2 and RWT <0,42.
Statistical analysis. Statistical processing was carried out using the statistical software package Statistica, v.12.0 (StatSoft, USA). The distribution of the studied parameters was carried out using the Kolmogorov-Smirnov test. Qualitative variables are presented as relative frequencies (n, %). Quantitative characteristics are presented as the interquartile interval median, Me [ 25%; 75%]. To confirm statistical significance, the Mann-Whitney U-test was used when comparing 2 independent groups. Spearman’s rank correlation coefficient was used to assess the relationship between the considered features. Data differences and correlations between data were considered significant at p<0,05.
Results
According to the ABPM, patients were divided into following groups: 1 — controlled (n=44) and 2 — uncontrolled (n=48) resistant HTN. The clinical and demographic characteristics of the patients are presented in Table 1. The groups were comparable in terms of cardiovascular risk factors. Assessment of target organ damage (Table 2) (decrease in renal function, LVMI, and intimamedia thickness) also did not reveal differences between the groups.
With a comparable duration of HTN, we found a longer history of regular antihypertensive therapy in the group of controlled resistant HTN (7,0 [ 4,0; 8,0] years in group 1 and 5,0 [ 4,0; 7,0] years in group 2, p=0,034). At entry into the study, 100% of participants received combined antihypertensive therapy with RAAS inhibitors, diuretics, calcium channel blockers, and an aldosterone receptor antagonist. In addition, 20,5% of patients in group 1 and 25% in group 2 took ?-blockers, while 22,7% and 27,1% of patients in groups 1 and 2, respectively, took an imidazoline receptor agonist. The average number of drugs in group 1 was 4,4 [ 4,0; 4,6], in group 2 — 4,4 [ 4,1; 4,6] (p=0,673). Thus, the groups were comparable in qualitative and quantitative composition of therapy, which makes it possible to exclude a direct drug effect on the studied parameters.
The study design involved the division of patients with resistant HTN into groups depending on the achievement of the target BP level according to ABPM results against the background of comparable multicomponent therapy. In the group of uncontrolled resistant HTN, the main parameters of ABPM were higher (Table 3). The heart rate and 24-hour diastolic BP index were comparable [19-23].
There was no significant difference between the groups (Table 4) in standard laboratory investigations, including the level of GFR, MAU, uric acid, microelements and lipid profile, despite the fact that before inclusion in the study, none of the patients had received statin therapy for 6 months. In the group of uncontrolled resistant HTN, the level of FGF23 was significantly higher — 11,7 [ 8,5; 15,4] pmol/ml vs 9,2 [ 7,1; 11,6] pmol/ml in group 1 (p=0,0036).
The echocardiographic data are presented in Table 5. The mean values of end-diastolic dimension, end-systolic dimension, end-systolic volume, end-diastolic volume, ejection fraction were within the normal range and did not differ in both groups of patients with resistant HTN. A comparable moderate increase in left atrial size and LV diastolic function impairment were found in the study of transmitral blood flow, regardless of the achievement of the target BP level. Also in both studied groups, a thickening of LV posterior wall and interventricular septum was revealed. At the same time, the greatest interventricular septum thickness values were found in patients of the 2nd group as follows: 1,3 [ 1,2; 1,4] cm vs 1,2 [ 1,1; 1,3] cm in group 1 (p=0,0043). But more significant differences were obtained when calculating the LV RWT: in group 1, LV RWT was 0,45 [ 0,43; 0,50], in group 2 — 0,50 [ 0,48; 0,53], p<0,0001. At the same time, LVM and LVMI were comparable.
Table 1
Clinical and demographic characteristics of patients (Ме [ 25%; 75%])

Abbreviations: HTN — hypertension, BMI — body mass index, WC — waist circumference.
Table 2
Assessment of target organ damage in patients with resistant HTN (Ме [ 25%; 75%])

Abbreviations: HTN — hypertension, LVMI — left ventricular mass index, MAU — microalbuminuria, GFR — glomerular filtration rate, IMT — intima-media thickness.
Table 3
ABPM data (Ме [ 25%; 75%])

Abbreviations: HTN — hypertension, MBPS — morning blood pressure surge, DBP — diastolic blood pressure, PP — pulse pressure, SBP — systolic blood pressure.
Table 4
Laboratory data of patients with resistant hypertension (Ме [ 25%; 75%])

Abbreviations: HTN — hypertension, HDL — high-density lipoprotein, LDL — low-density lipoprotein, MAU — microalbuminuria, UA — uric acid, GFR — glomerular filtration rate, TG — triglycerides, FGF23 — fibroblast growth factor-23.
Table 5
Echocardiographic data of patients with resistant hypertension (Ме [ 25%; 75%])

Abbreviations: HTN — hypertension, LVMI — left ventricular mass index, EDD — end-diastolic dimension, EDV — end-diastolic volume, ESD — end-systolic dimension, ESV — end-systolic volume, LV — left ventricle, LA — left atrium, LVM — left ventricular mass, RWT — relative wall thickness, PWT — posterior wall thickness, IVST — interventricular septal thickness, SV — stroke volume, EF — ejection fraction.

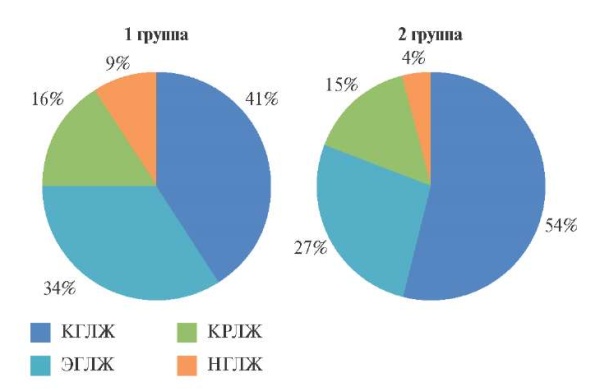
Figure 1. Types of LV geometry in patients with resistant hypertension.
Abbreviations: CLVH — concentric left ventricular hypertrophy, CLVR — concentric left ventricular remodeling, LVH — normal left ventricular geometry, ELVH — eccentric left ventricular hypertrophy.
When studying the type of LV geometry (Fi – gure 1), we found that the leading patterns in groups 1 and 2 were concentric LVH — in 18 patients of group 1 and 26 in group 2 (p=0,044), less often — eccentric LVH. Thus, in the majority of patients with resistant HTN, we found a change in LV shape from physiological ellipsoid to hemodynamically unfavorable spherical, thickening of LV walls with a regular increase in myocardial mass and the formation of predominantly hypertrophic LV remodeling.
To assess the relationship between the studied parameters, we conducted a correlation analysis, which found a positive relationship of pulse pressure (PP) with HTN duration (r=48, p=0,02) and FGF23 level (r=0,62, p=0,004). LVMI was positively associated with the time index for diastolic BP (r=51, p=0,02). A positive correlation was also found between LV RWT and PP (r=0,64, p=0,02) and a negative relationship with the duration of regular antihypertensive therapy (r=47, p=0,04). But the strongest relationship was found between LV RWT and FGF23 levels (r=0,75, p=0,005).
Discussion
The risk of LVH in patients with resistant HTN is significantly higher than in the general population of patients with HTN (odds ratio, 2,1, confidence interval, 1,2-3,6) [24]. As is known, LVH is a modifiable risk factor, and a decrease in LVM, in addition to a decrease in BP, is one of the essential criteria for the effectiveness of antihypertensive therapy. It is known that an intensive decrease in BP is accompanied by a more pronounced regression of LVH [25]. In the present study, patients with resistant HTN had elevated LVM and LVMI, regardless of the achievement of the target BP level. These results can be explained by the fact that long-term use of RAAS blockers and calcium channel antagonists leads to LVH regression, regardless of the achievement of the target BP level [17]. At the same time, we found significantly higher RWT values and more frequent development of the concentric LVH variant in patients with uncontrolled resistant HTN.
We also found higher PP values in patients with uncontrolled resistant HTN. This is consistent with the results of the Swedish Primary Care Cardiovascular Database (SPCCD) cohort study [26], in which a pattern of isolated systolic hypertension (ISH) was more frequently detected in patients with uncontrolled resistant HTN. It is known that ISH is currently considered as the main risk factor for CVEs in the elderly. The results of numerous studies have shown that the development of LVH and LV remodeling in ISH is associated not only with the level of systolic BP, but also with PP and the related vascular stiffness, which in this study is confirmed by the presence of a relationship between LV RWT and PP.
As already mentioned, FGF23 is a direct mediator of LVH development [10-12]. It was found that in patients with resistant HTN, LVM and LVMI were significantly higher with a higher level of FGF23 [17]. In this study, the level of FGF23 was higher in patients with uncontrolled resistant HTN, but we did not find an association with LVM. At the same time, FGF23 was associated with the PP value according to ABPM and LV RWT. The revealed relationships can be explained by the fact that FGF23 is one of the humoral regulators of vascular calcification [9]. In HTN, the level of FGF23 correlates with aortic calcification, which is known to make a significant contribution to the progression of vascular stiffness, an increase in heart afterload, and the development of LVH [24]. Increased vascular stiffness is a strong independent predictor of all CVEs in all categories of patients with cardiovascular pathology, and the most pronounced vascular changes were found in patients with resistant HTN, even when target BP was achieved against the background of antihypertensive therapy [24].
Study limitations. This cross-sectional singlecenter study was performed in a selected cohort of patients with resistant HTN without associated clinical conditions and clinically significant comorbidities, who received proper antihypertensive therapy and were regularly observed by specialists. In this regard, the features of myocardial remodeling identified and their relationship with the FGF23 level cannot reflect the structural and geometric myocardial changes in the general population of patients with resistant HTN. To evaluate the studied parameters in patients with resistant HTN, large multicenter studies are required.
Conclusion
For patients with uncontrolled resistant HTN, an increase in PP and myocardial remodeling in the form of concentric hypertrophy are more characteristic. FGF23 is significantly higher in uncontrolled resistant HTN and is positively associated with PP and RWT. Although these results need to be confirmed in a larger prospective study, these data suggest that FGF23 may play an important role in LV remodeling in patients with resistant HTN.
Relationships and Activities: none.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Litvinova M.S., Khaisheva L.A., Shlyk S.V. Myocardial remodeling and fibroblast growth factor in patients with resistant hypertension. Russian Journal of Cardiology. 2022;27(4S):5056. https://doi.org/10.15829/1560-4071-2022-5056
Copy