Predictors of atrial fibrillation recurrence in patients with metabolic syndrome after pulmonary vein isolation
Аннотация
Aim. To determine the role of biomarkers in predicting atrial fibrillation (AF) recurrence within 12 months after radiofrequency ablation (RFA) in patients with metabolic syndrome (MS).
Material and methods. The study included 245 patients with AF aged 35 to 65 years: patients without MS components (n=32), with 1-2 MS components (n=62) and patients with 3 or more MS components (n=153). All patients underwent a comprehensive clinical and anamnestic, anthropometric, laboratory and echocardiographic examinations. The prospective follow-up for 12 months included 135 patients with AF who underwent RFA.
Results. It was found that the presence of 3 or more MS components increased the risk of AF recurrence by 4,1 times within 12 months after RFA (relative risk (RR) =4,1, 95% CI 2,19-7,65, p<0,0001). According to binomial logistic regression, epicardial fat thickness (EFT) (OR =3,71, 95% CI 2,12-6,73, p=0,00001), the severity of left atrial fibrosis (OR =1,48, 95% CI 1,03-1,78, p=0,0006), concentrations of galectin-3 (OR =1,31, 95% CI 1,12-1,51, p=0,0001) and GDF-15 (OR =1,11, 95% CI 1,02-1,18, p=0,0002) in patients with AF and MS increase the risk of AF recurrence after RFA. For galectin-3, GDF-15, and EFT, using ROC analysis, the following threshold values were established, the excess of which had the greatest effect on the risk of AF recurrence after RFA in patients with MS: galectin-3 >11,0 ng/ml (RR =3,43, 95% CI 1,79-6,58, p=0,0001), GDF-15 >1380,7 pg/ml (RR =2,84, 95% CI 1,81-4,46, p<0,0001) and EFT >6,4 mm (RR =4,50, 95% CI 2,32-8,71, p<0,0001). In patients with excess of all three biomarker thresholds, the total risk of AF recurrence in patients with MS within 12 months after RFA increases by 3,2 times (RR =3,16, 95% CI 1,97-5,11, p<0,00001).
Conclusion. The risk of AF recurrence within 12 months after RFA in patients with three or more MS components is higher than in patients with 1-2 MS components. An increase in the blood concentration of profibrogenic biomarkers galectin-3, GDF-15 and an increase in the thickness of epicardial adipose tissue is associated with an increased risk of AF recurrence in patients with MS, and these biomarkers are likely to play a significant role in predicting recurrent episodes of AF after RFA.
Metabolic syndrome (MS) is a cluster of pathological conditions that includes arterial hypertension (AH), abdominal obesity (AO), insulin resistance, and lipid metabolism disorders that all contribute to the development of cardiovascular diseases [1]. The prevalence of MS in the population of the Russian Federation is high and, according to the epidemiological ESSE-RF study, it is accounted for 33% in those examined at the age of 25-64 years [2]. Putting into clinical practice the MS criteria of the Joint Interim Societies, the Joint Interim Statement (JIS 2009) allows more frequently identifying this pathology and timely preventing of cardiovascular diseases [3]. Atrial fibrillation (AF) is the most common type of heart arrhythmia associated with an increased risk of stroke, systemic embolism, disability and mortality in the working-age population [4]. The main conditions of MS, such as AO, AH, and carbohydrate metabolism disorders, significantly increase the risk of developing AF, including younger generation [5]. The mechanisms of AF development in patients with MS are manifold and are caused by structural changes in the atria, a process known as remodeling. Anatomical atrial remodeling is accompanied by atrial dilatation due to an increased volume of circulating blood in patients with AO and an elevated filling pressure in the left ventricle (LV) in patients with AH [6][7]. Impaired carbohydrate metabolism and diabetes mellitus (DM) jointly with visceral adipose tissue contribute to electrical and structural myocardial remodeling, which can lead to the formation of fibrosis. This, in turn, leads to the occurrence of micro-reentry as a substrate for the development of atrial fibrillation [8]. Recently the role of circulating biomarkers of fibrosis and inflammation has been actively studied in the manifestation of AF in various cohorts. Previously, increased galectin-3, procollagen types I and III expression has been revealed in patients with AF and MS, and the relationship between biomarkers and the severity of left atrial (LA) fibrosis has been established [9]. Epicardial adipose tissue, a biomarker of visceral obesity, promotes not only the systemic circulation of proinflammatory and profibrogenic cytokines, but also has a paracrine effect on the myocardium, which leads to the development of fibrosis and electrical remodeling [10]. Studying the role of various biomarkers exists due to the need to find out predictors not only for the risk of developing AF, but also for the progression of this arrhythmia, as well as for the effectiveness of drug and interventional therapy. According to the results of the study, it was found that elevated concentrations of galectin-3 and aldosterone was associated with an increased risk of recurrence of this rhythm disorder in patients with AF after radiofrequency ablation (RFA) [11]. In 2012, the results of a retrospective study were published, where MS was identified as a significant predictor of AF recurrence after RFA (relative risk (RR) =1,28, p=0,021). However, a comprehensive study of the role of various biomarkers has not been carried out yet [12]. The objective of this study was to evaluate the role of clinical, anthropometric, profibrogenic and pro-inflammatory factors as predictors of AF recurrence in patients with MS after RFA.
Material and methods
From 2014 to 2018, 245 (male of 55,9% and female of 44,1%, at the age of 35-65 years) patients out of 1307 patients with AF hospitalized in the therapeutic clinic of Federal State Budgetary Educational Institution of Higher Education “Academician I.P. Pavlov First St. Petersburg State Medical University” of the Ministry of Healthcare of Russian Federation were enrolled. The patients did not have any organic heart disease, clinical data for acute and chronic diseases. The study was approved by the local Ethics Committee of Institution. Informed written consent was obtained from all patients before their enrollment. The study included patients with paroxysmal (n=191) and persistent (n=54) forms of AF and a different number of MS components (JIS, 2009): no components or control group (n=30), 1-2 components (n= 62) and ?3 components (n=153). All the examined patients were assessed the anthropometric data, a set of laboratory and instrumental examinations: electrocardiogram (ECG), multi-day ECG monitoring (“Normocard”, Kemerovo, Russia), echocardiography (“Vivid 7”, GE, USA) with evaluation of epicardial fat thickness (EFT). All patients who underwent RFA received cardiorespiratory monitoring to find oud sleep-related respiratory disorders (SOMNOlab 2 (PG) Polygraphy system, Loewenstein Medical, Weinmann, Germany). All plasma and serum samples were centrifuged with subsequent storage at -40? C. The levels of biomarkers were determined using standard kits; their data are presented in Table 1.
The prospective 12-month follow-up included patients with clinically significant AF paroxysms (involving those with reduced quality of life due to this condition), resistant to drug antiarrhythmic therapy, who underwent pulmonary vein isolation ablation.
Scheduled outpatient visits after RFA were carried out at 3, 6, 9 and 12 months. AF recurrence after RFA was considered to be complaints of patients and recorded episodes of AF ?30 sec on ECG in the period from 3 to 12 months. All patients underwent 3-day ECG monitoring at 6 months after RFA and longer up to 7-day ECG monitoring at 12 months after RFA using the Normocard system (Kemerovo) to reveal/exclude AF paroxysms after the RFA procedure, which was considered effective in the absence of complaints and indications of AF paroxysms in the period from 3 to 12 months. Against the background of sinus rhythm, bipolar amplitude LA maps and local activation time estimation maps were performed before RFA of the pulmonary vein in an X-ray operating room using a non-fluoroscopic electrical and anatomical mapping system CARTO 3 (Biosense Webster, USA) and a catheter measuring the contact force with the LA myocardium (Smart Touch Thermocool, Biosense Webster, USA). The assessment of low voltage zones in the amplitude spectrum of 0,2-0,5 mV with the measurement of their area using the “area measurement” function of the CARTO 3 navigation system software was carried out in the “off line” mode [13]. The prevalence of fibrosis was assessed as a percentage of the area of fibrosis to the total area of the LA.
Statistical analysis was performed using licensed IBM SPSS Statistics software, version 22.0. The Kolmogorov-Smirnov test was carried out to evaluate the normality of the distribution. Normally distributed quantitative variables are presented as mean (M) ± standard deviation (?). The parametric unpaired Student’s t-test was used to compare the normally distributed quantitative variables of independent groups. Abnormally distributed quantitative variables are presented as medians (Me) with interquartile intervals (25-75%), and the nonparametric Mann-Whitney U-test was used to compare such variables in independent groups. The parametric analysis of variance (ANOVA) and the non-parametric Kruskal-Wallis test were used for multiple comparisons in groups (more than two). Binomial regression analysis was also used to predict the probability of event occurrence (odds ratio (OR)) and ROC-analysis to determine the cut-off value of biomarkers with the relative risk of events (RR) using a 2?2 contingency table.
Table 1
Characteristics of methods and manufacturers of reagents for the evaluation of biomarker levels in blood samples

Abbreviations: IL-6 — interleukin-6, IT — immunoturbidimetry, ELISA — enzyme-linked immunosorbent assay, CRP — С-reactive protein, TNF-? — tumor necrosis factor alpha, TGF-beta1 — transforming growth factor-beta1, CT-1 — cardiotrophin-1, CTGF — Connective tissue growth factor, PIIINP — N-terminal propeptide of procollagen type III, PINP — N-terminal propeptide of procollagen type I, GDF15 — growth differentiation factor-15.
Results
Patients with AF without MS components and with varies numbers of MS components were included. The studied groups were comparable by sex and did not differ statistically significantly by age. Patients with 1-2 MS components had higher body mass index (BMI) and waist circumference (WC) than those without MS components. However, these groups did not differ significantly in terms of lipid and carbohydrate metabolism parameters. The highest values of BMI, WC, and plasma glucose concentrations were found in patients with ?3 MS components, but lipid metabolism parameters were comparable with the comparison groups. Patients with AF and MS had significantly larger sizes of the left and right atria, greater values of the LV myocardial mass index (LVMMi) and EFT than patients with 1-2 components of MS and patients without MS. Bothe he dimension and volume of the LA and LVMMi were greater in patients with 1-2 MS components than in patients without MS. The examined groups were comparable in terms of LV ejection fraction and duration of AF. The main clinical, laboratory and instrumental characteristics of the examined patients are presented in Table 2.
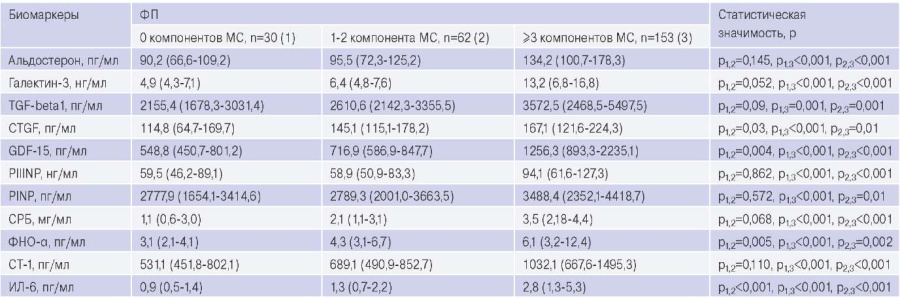
Table 3 presents data on the serum and plasma biomarker concentrations of the patients. The levels of pro-fibrogenic and pro-inflammatory biomarkers have been established to be higher in patients with AF and MS than in those with AF and 1-2 MS components or without MS components. The concentrations of connective tissue growth factor, growth differentiation factor-15 (GDF-15), tumor necrosis factor alpha and interleukin-6 were greater in patients with AF and 1-2 components of MS than in patients with AF and without MS.
The further prospective 12-month follow-up observation included patients with indications for RFA (n=135) without MS and various numbers of MS components: 0 MS components (n=23), 1-2 MS components (n=35), ?3 MS components (n=77). It was found that 42,9% of patients (n=58) had AF recurrence during follow-up period after RFA in the following ratio: 72,4% (n=49) of patients with ?3 MS components, 8,6% (n=5) of patients with 1-2 MS components and 6,9% (n=4) of patients without MS components. There were fewer patients with AF recurrence in those without MS components, than patients without recurrent AF episodes, and a low risk of AF recurrence after RFA was revealed (RR =0,34, 95% confidence interval (CI) 0,14-0,83, p=0,007). Patients with 1-2 MS components showed a trend towards an increased risk of AF recurrence, however, no statistically significant difference was found in comparison to patients without MS components (RR =0,82, 95% CI 0,21-3,25, p=0,779). In patients with ?3 MS components, there were more patients with recurrent AF than those without arrhythmia. Thus, the presence of MS increased the risk of AF recurrence by 4,1 times after RFA within 12 months (RR =4,11, 95% CI 2,19-7,65, p<0,0001). When analyzing the risk of AF recurrence after RFA in the examined patients based on the number of MS components, a statistically significant increase was observed in this parameter in patients with 3 or more components, and the maximum risk of recurrent episodes of AF after RFA was found in patients with 5 MS components (Table 4).
On binomial logistic regression, an increase in the number of MS components from 0 to 5 raised the likelihood of AF recurrence after RFA by 2,2 times (OR =2,16, 95% CI 1,61-2,89, p<0,0001). Data from patients with AF and 3 or more MS components underwent RFA were analyzed in more detail. Groups of patients with MS and AF with AF recurrence and without it after RFA were comparable distribution in age, BMI and sex. Having analyzed statistical data, WC in patients with recurrent AF has been found to be greater than in patients with effective RFA. Plasma triglyceride levels in patients with recurrent AF were higher than in patients without recurrent episodes of arrhythmias. Generally, the groups were comparable in terms of the prevalence of AO, AH, obstructive sleep apnea syndrome, but the frequency of DM in patients with recurrent AF after RFA was higher than in patients with the effective interventional therapy. The groups were matched for antiarrhythmic therapy after RFA. The patients received the following drugs: beta-blockers (32,6%), propafenone (31,9%), sotalol (25,9%), amiodarone (7,4%), allapinin (2,2%). It should be noted that the parameters characterizing the dilatation of both atria were comparable in groups. However, LVMMi was higher in patients with recurrent AF after RFA. EFT and the severity of LA myocardial fibrosis were significantly higher in patients with recurrent episodes of AF after interventional therapy than in patients without it (Table 5).
On binomial logistic regression, greater values of EFT (OR =3,71, 95% CI 2,12-6,73, p=0,00001) and significant fibrosis (OR =1,48, 95% CI 1,03-1,78, p=0,0006) in patients with AF and MS increased the risk of recurrence of this arrhythmia after RFA. Having analyzed the concentrations of biomarkers of fibrosis and inflammation, it was found that the levels of galectin-3 and GDF-15 were higher in patients with AF and MS with recurrent AF after RFA than in patients with no recorded recurrent episodes of AF. The concentrations of other profibrogenic and pro-inflammatory cytokines did not differ significantly in the groups (Table 6).
On binomial logistic regression, the levels of galectin-3 (OR =1,31, 95% CI 1,12-1,51, p=0,0001) and GDF-15 (OR =1,11, 95% CI 1,02-1,18, p=0,0002) in patients with MS increased the likelihood of AF recurrence within 12 months after RFA. For galectin-3, GDF-15, and EFT, ROC-analysis was used to calculate the predictive curves for the probability of AF recurrence in patients with MS within 12 months after RFA. The analysis revealed high areas under the curves, which correspond to a significant effect of these biomarkers on the likelihood of recurrence of AF after RFA in patients with MS (Figure 1). Based on ROC-analysis, the elevated levels of galectin-3 (>11,0 ng/mL; RR =3,43, 95% CI 1,79-6,58, p=0,0001), GDF-15 (>1380,7 pg/mL; RR =2,84, 95% CI 1,81-4,46, p<0,0001) and EFT (>6,4 mm; RR =4,50, 95% CI 2,32-8,71, p<0,0001) had the greatest effect on the risk of AF recurrence after RFA in patients with MS. If there was the excess of all three biomarker cut-off values, the total risk of AF recurrence in patients with MS within 12 months after RFA increased by 3,2 times (RR =3,16, 95% CI 1,97-5,11, p<0,00001). Sensitivity (Se — 75,5%) and specificity (Sp — 96,4%) indicated the high significance of this model for predicting the probability of the event.
Table 2
Clinical, laboratory and echocardiographic characteristics of the examined patients

Abbreviations: BMI — body mass index, LVMMi — left ventricular myocardial mass index, LA — left atrium, HDL — high density lipoproteins, LDL — low density lipoproteins, MS — metabolic syndrome, WC — waist circumference, RA — right atrium, TG — triglycerides, LVEF — left ventricular ejection fraction, AF — atrial fibrillation.
Table 3
Circulating blood levels of biomarkers of fibrosis and inflammation in patients with AF and MS, AF without MS, AF with 1-2 MS components

Abbreviations: IL-6 — interleukin-6, MS — metabolic syndrome, CRP — С-reactive protein, TNF-? — tumor necrosis factor alpha, TGF-beta1 — transforming growth factor-beta1, CT-1 — cardiotrophin-1, CTGF — Connective tissue growth factor, PIIINP — N-terminal propeptide of procollagen type III, PINP — N-terminal propeptide of procollagen type I, GDF-15 — growth differentiation factor-15.
Table 4
Risk of AF recurrence 12 months after RFA in patients with different numbers of MS components

Abbreviations: CI — confidence interval, MS — metabolic syndrome, AF — atrial fibrillation, RR — relative risk.
Table 5
Clinical, anthropometric, laboratory and echocardiographic characteristics of patients with AF and MS with and without AF recurrence after RFA

Abbreviations: ADT — antiarrhythmic drug therapy, ACE inhibitors/ARA — angiotensinconverting enzyme inhibitors/angiotensin receptor antagonists, LVMMi — left ventricular myocardial mass index, BMI — body mass index, LA — left atrium, HDL — high density lipoprotein, LDH — low density lipoprotein, RA — right atrium, RFA — radiofrequency ablation, OSAS — obstructive sleep apnea syndrome, TG — triglycerides, LV EF — left ventricular ejection fraction, AF — atrial fibrillation.
Table 6
Circulating blood levels of biomarkers of fibrosis and inflammation in patients with AF and MS with and without AF recurrence after RFA

Abbreviations: IL-6 — interleukin-6, MS — metabolic syndrome, CRP — С-reactive protein, TNF-? — tumor necrosis factor alpha, AF — atrial fibrillation, TGF-beta1 — transforming growth factor-beta1, CT-1 — cardiotrophin-1, CTGF — Connective tissue growth factor, PIIINP — N-terminal propeptide of procollagen type III, PINP — N-terminal propeptide of procollagen type I, GDF-15 — growth differentiation factor-15.

Figure 1. ROC curves of the blood levels of galectin-3 and GDF-15 and EFT for predicting the probability of AF recurrence in patients with MS within 12 months after RFA.
Abbreviations: EFT — epicardial fat thickness, AUC — area under the curve, GDF-15 — growth differentiation factor-15.
Discussion
AF is the most common arrhythmia worldwide. It is expected that the incidence of AF will increase significantly over the next few decades [14]. The mechanisms of development of this heart rhythm disorder are diverse and represent a whole system of changes, including electrical and structural remodeling with the formation of myocardial fibrosis, anatomical remodeling with the development of atrial dilatation and impaired contractile function, as well as changes in neurovegetative regulation [15]. MS components, such as hypertension, obesity, disorders of carbohydrate and lipid metabolism, are among the risk factors for the development of AF. The presence of a large number of MS components significantly increases the likelihood of developing this arrhythmia [16]. Sinus rhythm control with drug antiarrhythmic therapy and interventional treatments plays a substantial role in the up-to-date mana – gement strategy of patients with AF. Data from the prospective EAST-AFNET study showed that early control of sinus rhythm in patients with AF can reduce the risk of cardiovascular death and stroke, which makes antiarrhythmic drug therapy of patients with AF even more relevant [17]. Nevertheless, these facts such little amount of antiarrhythmic drugs, their insufficient effect or cardiovascular and other system adverse events limit the choice of drug therapy [18]. Current techniques of interventional treatment of AF, including, in particular, radiofrequency pulmonary vein isolation ablation make it possible to control sinus rhythm with high efficiency for a long time to a greater extent in patients with paroxysmal AF. However, this method of treatment has a rather high efficiency even in patients with a persistent form. The results of the CABANA study found that catheter ablation in AF was more effective in terms of sinus rhythm control compared to medical therapy, and both tactics were comparable in the risk reduction of cardiovascular complications [19]. In turn, the choice of antiarrhythmic tactics always requires personalized strategy, taking into account comorbid conditions. According to numerous studies, it has already been established that obesity, diabetes, uncontrolled hypertension can increase the risk of arrhythmia recurrence after RFA, which is probably due to persistent pathogenetic changes in structural remodeling and progressive myocardial fibrosis [12][20].
One of the largest meta-analyses, including 23 studies and 12924 patients with AF, found that MS increased the risk of AF recurrence (RR =1,63, 95% CI 1,25-2,12) [21]. In our cohort study [20], it was revealed that the presence of 3 or more MS components increased the risk of AF recurrence after RFA by 4,1 times (RR =4,11, 95% CI 2,19-7,65, p<0,0001). It was proved that the increase in the number of MS components from 0 to 5 raised the risk of AF recurrence by 2,2 times. The obtained data confirmed the fact that worsening of RFA efficiency prediction was observed only in the occurrence of 3 or more MS components, i.e. in the presence of criteria for this syndrome. It is worth noting that the examined groups of patients with MS and recurrent AF were comparable in terms of the occurrence of AH. However, in patients with no effect of interventional therapy, DM and AO were more common.
The risk stratification of AF recurrence in patients with MS seems to be extremely relevant, because identification of predictors of insufficient effect of RFA will allow personalizing the strategy for managing patients with AF. In recent years, various biomarkers affecting myocardial fibrosis have been actively studied. It was previously established that the concentrations of such biomarkers as galectin-3, transforming growth factor-beta1, N-terminal propeptide of procollagen type I and III is increased in patients with MS and AF and are associated with the severity of LA myocardial fibrosis [22]. It has been found that the blood circulating levels of GDF-15 are associated with the severity of myocardial fibrosis and are likely to have an important prognostic value for patients with AF [23]. It was revealed that the increase in the concentrations of galectin-3 and aldosterone is associated with the increased risk of recurrence of this heart rhythm disorder in patients with AF in the study of biomarkers of fibrosis and inflammation in patients with AF and RFA [11].
Elevated serum levels of transforming growth factor-beta1 was an independent predictor of AF recurrence after RFA (OR =1,14, 95% CI 1,11-1,17, p=0,02) [24]. However, to date, studies on the comprehensive assessment of the role of biomarkers of fibrosis and inflammation in the development of AF relapses after RFA in patients with MS have not been published. However, to date, studies on the comprehensive assessment of the role of biomarkers of fibrosis and inflammation in the development of AF relapses after RFA in patients with MS have not been published. However, to date, studies on the comprehensive assessment of biomarkers of fibrosis and inflammation in the development of AF recurrence after RFA in patients with MS have not been published. It is worth noting that our examined patients with MS and AF had higher values of a large number of fibrosis biomarkers studied, in comparison with patients with AF and with 1-2 MS components. The peculiarity of our study is that patients with MS in the groups with and without AF recurrence were comparable not only in age, BMI, duration of AF and received drug therapy, but they also did not differ significantly in the severity of both atria dilatation. Simultaneously, echocardiography showed a significantly higher EFT values in patients with recurrent AF after RFA, and, on binomial regression analysis, this biomarker was found to increase the risk of arrhythmia recurrence (OR =3,71, 95% CI 2,12-6,73, p=0,00001) [25][26]. It is known that MRI and CT are the gold standard for imaging EFT assessment; however, echocardiography is a more accessible screening method for evaluating this parameter in real clinical practice. In our study, based on ROC analysis, a cut-off value of EFT of 6,4 mm was revealed. Exceeding this cut-off significantly increases the risk of AF recurrence after RFA (RR =4,50, 95% CI 2,32-8,71, p<0,0001). A greater increase in EFT in patients with recurrent AF after RFA might be associated with a more severe the degree of fibrosis with a comparable volume of LA in the comparison groups with MS. In turn, the severity of LA fibrosis increased the risk of AF recurrence in patients with MS (OR =1,48, 95% CI 1,03-1,78, p=0,0006). The study of pro-inflammatory and profibrogenic biomarkers revealed higher concentrations of galectin-3 and GDF-15 in patients with MS and recurrent AF. In recent years, various studies, including a large meta-analysis, have confirmed the association of galectin-3 with the risk of developing AF and myocardial fibrosis [27]. Pro-inflammatory cytokines activate macrophages and increase the production of galectin-3. Overexpression of galectin-3 induces fibroblasts to increase the secretion of collagen into the intercellular space, and therefore the highest concentrations of this biomarker are observed in patients with obesity and DM [28, 29]. It was found that the plasma concentration of galectin-3 >5,83 ng/mL in patients with persistent AF without structural heart disease increases the risk of arrhythmia recurrence after RFA (RR =1,28, 95% CI 1,072-1,529, p<0,006). In our cohort study, we studied the role of galectin-3 in predicting the risk of AF recurrence, predominantly in patients with paroxysmal arrhythmia in combination with MS. We also established the prognostic role of this biomarker, and the cut-off value of galectin-3 >11,0 ng/mL increased the risk of recurrence by more than 3 times (RR =3,43, 95% CI 1,79-6,58, p=0,0001). It can be assumed that the production of galectin-3 is significantly increased in the early stages of myocardial fibrosis formation. Nevertheless, given its significant prognostic role in patients with not only paroxysmal, but also persistent forms of AF, it is possible to believe that this biomarker acts as a predictor of more long-term prognosis in patients with AF.
As our study showed, the biomarker GDF-15 is no less important in terms of predicting AF recurrence after RFA in patients with MS. The results of numerous studies have established that this biomarker increases in inflammation, oxidative stress, hypoxia, and hyperglycemia. It probably determines its important integrative role as an indicator of adverse metabolic pathological processes in the body [30]. In patients with AF, the prognostic role of GDF-15 in the risk stratification of bleeding was well-studied in a large randomized trial ARISTOTLE, where GDF-15 was proved to be associated with the risk of cardiovascular death and bleeding [31]. In 2020, data on the role of GDF-15 in predicting AF recurrence was firstly published. It was found that exceeding the cut-off value of 1287,3 pg/ml increased the risk of AF recurrence (RR =1,053, 95% CI 1,007-1,1, p=0,022), however, this predictive model had low sensitivity (51,4%) and specificity (70,8%), and more than half of the patients in the study had persistent AF [32]. According to the results of our study, the GDF-15 biomarker also demonstrated a high prognostic role in predicting AF recurrence after RFA in a cohort of patients with MS, wherein the cut-off value of GDF15 was higher (1380,7 pg/ml), and the increase in the risk of recurrence was found to be more degree (RR =2,84, 95% CI 1,81-4,46, p<0,0001).
The use of various biomarkers in actual clinical practice allows personalization of therapy to achieve a better result in reducing the risk of endpoint events. In particular, a comprehensive assessment of such prognostic biomarkers as galectin-3, GDF-15, and EFT in patients with AF and MS makes it possible to predict AF recurrence within 12 months after RFA with a high degree of sensitivity and specificity. The main practical significance of such personalized therapy is the timely identification of patients with an unfavorable prognosis of RFA. In those patients primary prevention such as the impact on risk factors of AF: weight loss, normalization of blood pressure and stabilization of the glycemic profile, identification and correction of respiratory disorders during sleep in preparation for an intervention therapy should be intensified. All that may improve the prognosis of the effectiveness of radiofrequency pulmonary vein isolation in patients with MS.
Conclusion
1. The presence of 3 and more MS components increases the risk of AF recurrence by 4,1 times within 12 months after RFA, and the highest risk of recurrent episodes of this arrhythmia was found in patients with 5 MS components.
2. On binomial logistic regression, independent predictors of AF recurrence such as EFT, severity of LA fibrosis, elevated blood levels of galectin-3 and GDF-15 were identified in patients with MS during the first 12 months after RFA.
3. Cut-off values of the blood concentrations of galectin-3 (>11,0 ng/mL), GDF-15 (>1380,7 pg/ mL) and EFT (>6,4 mm) have been established. The excess of those significantly affects the risk of AF recurrence after RFA in patients with MS.
Relationships and Activities: none.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Ionin V.A., Zaslavskaya E.L., Barashkova E.I., Pavlova V.A., Ananev A.M., Morozov A.N., Baranova E.I. Predictors of atrial fibrillation recurrence in patients with metabolic syndrome after pulmonary vein isolation. Russian Journal of Cardiology. 2022;27(3S):5184. https://doi.org/10.15829/1560-4071-2022-5184
Copy