Polymorphism of ACE, AGT, AGTR1 genes as genetic predictors of hypertension
The genetic architecture of blood pressure (BP) currently includes >30 genes, including genes with rare mutations leading to hereditary forms of hypertension or hypotension, and 1477 polymorphic variants. This determines the phenotypic heterogeneity of BP and corresponds to Page’s mosaic theory, according to which the development of essential hypertension (HTN) is associated with a complex of interrelated disorders in various systems: hemodynamic, metabolic, neurohumoral. This theory considers essential HTN not as a single disease, but as a combination of diseases (subtypes of essential HTN) differing to one degree or another with different origins, development and consequences. The mosaic of HTN causes, if it exists for essential hypertension, needs clarification, since it potentially opens up new opportunities for stratification, the development of new drugs, and personalized medicine [1][2].
General view of single nucleotide polymorphism. The most common cause of differences in gene structure is point mutations — single-nucleotide polymorphism (SNP), which is the replacement of one nitrogenous base with another in a DNA or RNA region, leading to the appearance of a particular phenotypic trait. A large number of studies confirm that SNPs can contribute to the predisposition to some diseases, in particular to HTN [3]. However, a certain polymorphism is not always associated with the certain phenotypic trait. Currently, the phenomenon of pleiotropy is known, which means that the same polymorphism can have several phenotypic manifestations. For example, a genetic predisposition to smoking is associated with 361 polymorphic variants in 14 genes involved in the development of cardiovascular pathology [4]. These phenotypic differences can be due to various reasons, including interaction of different SNPs [5][6].
In clinical practice, genetic analysis is more often carried out using molecular testing of candidate genes for disease susceptibility. These are genes, hereditary (polymorphic) variants of which, to a relatively small extent affecting the encoded proteins (enzymes), in combination with unfavourable external factors, can cause various diseases [7].
Given that a human’s genetic information is largely stable from birth, it can act as an early predictor of HTN. Identification of polymorphic variants of genes associated with a high risk of HTN can be one of the promising areas of early diagnosis and prevention of this disease. In addition, the availability of this information will clarify the prognosis of people already suffering from this disease, as well as personalize the approach to patient treatment [1][3][7][8][9][10]. Such a personalized approach to treatment, based on data on various effects of drugs, depending on the genome of a particular individual, is the most important task of pharmacogenomics. It should be noted that, despite the progress achieved in this field in recent years, there are still no official guidelines on a personalized approach that takes into account the pharmacogenomics of antihypertensive drugs. However, for example, regarding oral anticoagulants and some anticancer drugs, such guidelines were developed.
Twin studies have shown that the heritability of hypertension is 40%. To assess the heritability of BP, American scientists used genomic polymorphism data from the Atherosclerosis Risk in Communities (ARIC) study. According to this paper, heritability was 20%/50% and 27%/39% for systolic/diastolic BP in individuals of European and African descent, respectively [11].
The profile of candidate genes involved in HTN is quite wide and includes groups of genes that control various metabolic and homeostatic systems. In particular, there are genes of the renin-angiotensinaldosterone system (RAAS) (angiotensinogen (AGT) gene, renin gene, angiotensin-converting enzyme (ACE) gene, angiotensin-II receptor type 1 gene (AGTR1), etc.), genes for lipid metabolism (apolipoprotein A1 gene, apolipoprotein B gene, apolipoprotein C gene, apolipoprotein E gene, lipoprotein lipase gene, etc.), and genes determining the vascular endothelium status (endothelial nitric oxide synthase gene, endothelin gene, paraoxonase gene, etc.) [3][12].
The review analyses the works devoted to the molecular genetic basis of HTN and identifies the possible role of RAAS genes’ polymorphism. The literature review included following works: reports of randomized and cohort studies on large populations; meta-analyses and systematic reviews; papers in English and Russian.
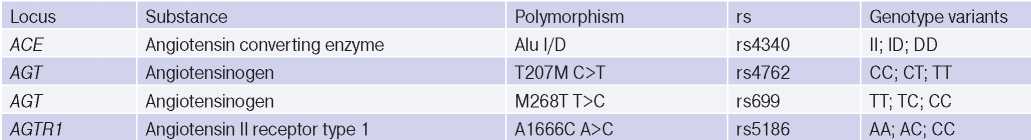
Role of some polymorphic variants of ACE, AGT, AGTR1 genes in HTN development. Dysfunction of the RAAS plays a leading role in HTN pathogenesis. The activity of this system is to a certain extent determined genetically and depends on polymorphism of ACE, AGT, AGTR1 genes [3]. Characteristics of polymorphic variants of these genes are presented in Table 1. It should be noted that in recent years there is information about a relatively small number of studies devoted to relationship between the polymorphism of these genes and a predisposition to HTN. The most studied is the ACE gene rs4340 (Alu I/D) polymorphism.
Table 1
Characteristics of the studied polymorphic variants
Clinical value of Alu I/D (rs4340) polymorphism of ACE gene. The ACE gene is located on chromosome 17 (17q23.3) and is responsible for ACE synthesis, which plays an important role in the regulation of BP and electrolyte balance. ACE is secreted from the lung and renal endothelial cells and promotes the conversion of angiotensin I to angiotensin II (AT-II), which is a powerful vasopressor and aldosterone stimulating substance. In addition, the enzyme is capable of inactivating bradykinin, which acts as a vasodilator [4]. The influence of the ACE gene has been well studied, and most of the published data refer to the rs4340 (Alu I/D) polymorphism leading to the insertion (I) or deletion (D) of the 289 bp Alu repeat sequence, affecting serum and tissue ACE levels. In individuals with a carrier of allele II, it is minimal, and in individuals with the DD allele — maximal [8]. A large number of studies have revealed the relationship of the DD variant with HTN [12][13][14][15].
A Chinese study found that ACE Alu I/D polymorphism was associated with in-stent restenosis in patients with coronary artery disease (CAD) after percutaneous coronary intervention and intracoronary drug-eluting stent implantation [16].
In addition, one of the studies conducted among children and adolescents reported a higher risk of high BP in individuals with genotype ID, especially among boys [15]. There is also information about the association of this polymorphic variant with atherosclerosis, CAD, myocardial infarction [13][17][18]. Thus, the Polish study found that in patients with CAD, the ACE gene D allele was significantly more frequent, which was also associated with increased levels of total cholesterol, low-density lipoprotein cholesterol [19]. In addition, there is evidence of an increased risk of diabetic nephropathy in individuals with ID/DD allele [20].
There is a genetic hypothesis about the participation of this gene polymorphism in the pathogenesis of coronavirus disease 2019 (COVID-19), determining the severity of the clinical course. It was found that the ACE gene D/D polymorphism in the genotype is associated with a more severe course and increased mortality among the Caucasian race. Most likely, this is due to the RAAS hyperactivation after SARS-CoV-2 infection, mainly due to virus and ACE-2 interaction and thus penetrating the target cells [21].
Clinical value of T207M C>T (rs4762) and M268T T>C (rs699) polymorphism of AGT gene. The AGT gene is located on chromosome 1 (1q42). This gene encodes the angiotensinogen, serum globulin produced by liver cells, from which, under the action of renin, AT-II precursor angiotensin I is formed, which is a strong vasopressor. To date, >15 polymorphic variants of the AGT gene are known, most of which lead to amino acid substitutions [22].
The following polymorphic variants of the AGT gene are associated with blood angiotensinogen level: T207M C>T (rs4762) — replacement of the cytosine nucleotide with thymine, leading to the replacement of the amino acid threonine with methionine at position 207 of the protein; М268Т Т>С (rs699) — replacement of the thymine nucleotide with cytosine, leading to the replacement of the amino acid methionine with threonine at position 268 of the
protein. Among population, the prevalence of these polymorphic variants of the AGT gene is 34-43%. The presence of risk alleles 207M and 268T of this gene is associated with an increased level of angiotensinogen expression and HTN development [23]. In addition, there are data on the relationship of the 207M and 268T alleles with other cardiovascular diseases. In particular, the M268T (rs699) polymorphism of the AGT gene is significantly associated with increase in CAD risk [24][25][26].
The presence of risk alleles 207M and 268T was also associated with the atrial fibrillation according to the Xinjiang (China) study [27].
Moreover, the M268T T>C polymorphism of the AGT gene was associated with an increased cardiovascular risk in patients with acromegaly [28].
Also, the 268T polymorphism of the AGT gene determines the effectiveness of ACE inhibitors in the treatment of HTN and congestive heart failure [29]. There is evidence of the association of this polymorphism with renal tubular dysgenesis, portal hypertension in patients with hepatitis C, and diabetic nephropathy in Asians [30][31][32].
These polymorphic variants of the AGT gene were also associated with vascular complications during pregnancy and hormone replacement therapy (since the AGT gene expression increases in response to ethinylestradiol action) [33][34][35][36][37].
Clinical value of A1166C A>C (rs5186) polymorphism of AGTR1 gene. The AGTR1 gene is localized on chromosome 3 (3q24) and encodes type I receptors for AT-II, located in the vascular endothelium and mediating all the main cardiovascular effects of angiotensin. Like other RAAS components, this gene is involved in BP regulation [4]. More than 50 of its polymorphic variants are known. The greatest clinical significance is the A1166C A>C (rs5186) polymorphism. In this case, the adenine nucleotide is replaced by cytosine at position 1166 of the DNA.
The presence of the C risk allele in the A1666C A>C polymorphism leads to an increased sensitivity of type 1 receptors to the normal AT-II level and, consequently, to higher BP. The prevalence of this polymorphism among the Caucasian race is quite wide and amounts to 27%. Studies have shown that hypertensive people were significantly more likely to have the A/C or C/C genotype of the AGTR1 gene compared with healthy people [38][39][40].
This polymorphism is associated with a change in AGTR1 gene expression regulation through interaction with miR155, which is a noncoding RNA molecule capable of complementary binding to untranslated regions of the target mRNA. MiR155 negatively regulates the expression of the AGTR1 gene, which increases protein synthesis and is associated with HTN [41]. In addition, there are 3 more aspects of AGTR1 regulation. First, activation of AGTR1 decreases the amount of the receptor in the cell. Secondly, long-term stimulation of AT-II decreased the production of AT-II through protein kinases. Third, there is modulation of AGTR1 gene expression [42].
It has also been shown that the AGTR1 gene plays an important role in atherogenesis. In the study with patients with occlusive peripheral arterial disease, it was found that carriers of AGTR1 gene CC genotype have significantly higher levels of low-density lipoprotein cholesterol (p=0,034) and triglycerides (p=0,007) [34]. The Chinese study showed that the AGTR1 gene AC genotype may be an additional independent risk factor for drug-eluting stent restenosis in patients with CAD over 60 years of age [16].
One of the most important factors contributing to the implementation of this or that genetic information is epigenetic influence. One of the epigenetic mechanisms of gene expression regulation is DNA methylation, which is the methylation of cytosine to 5-methylcytosine, primarily at CpG dinucleotides [43]. The recent study showed that AGTR1 methylation levels were significantly associated with CAD risk in men, suggesting sex-dependent effects in CAD pathogenesis. It has been shown that AGTR1 hypermethylation is associated with CAD risk in men, but not in women [44].
The study was also carried out on the relationship between the characteristics of intrarenal blood flow and the AGTR1 gene A1166C polymorphism in patients with grade 1-2 essential HTN and stage I-III chronic kidney disease (CKD). A decrease in systolic, diastolic, and averaged maximum blood flow velocities and an increase in blood flow acceleration time were found in patients with a higher stage of CKD, which may indicate an increased risk of early CKD development in patients with grade 1-2 essential HTN and AGTR1 gene 1166C risk allele [45].
Conclusion
The presented data suggests an important and undoubted role of RAAS genes’ polymorphism in HTN development. In addition, it was shown that the studied polymorphic variants of the RAAS genes are involved in the development of atherosclerosis and related diseases, disorders of the kidneys and central nervous system, the vascular complications during pregnancy and hormone replacement therapy, and justifies the effectiveness of ACE inhibitors in HTN therapy (Table 2). Most of the studies are devoted to the influence of any one gene polymorphism on BP level. Considering that the contribution of each of them individually is relatively small, it is obvious that accurate prediction of HTN risk will be possible by studying the cumulative impact of these multiple variants. This is done by means of a polygenic risk score (PRS), which is a mathematical disease risks’ combination associated with all SNPs involved in the regulation of BP [1]. In this regard, further study of the joint effect of several polymorphic variants of the RAAS genes and other systems involved in BP regulation on HTN seems to be very promising.
Table 2
Clinical value of the studied polymorphic variants

Abbreviations: ACE — angiotensin-converting enzyme, CAD — coronary artery disease.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Elkina A.Yu., Akimova N.S., Shvarts Yu.G. Polymorphism of ACE, AGT, AGTR1 genes as genetic predictors of hypertension. Russian Journal of Cardiology. 2021;26(1S):4143. (In Russ.) https://doi.org/10.15829/1560-4071-2021-4143
Copy