Prognostic value of left ventricular global longitudinal strain and mechanical dispersion by speckle tracking echocardiography in patients with ischemic and nonischemic cardiomyopathy: a systematic review and meta-analysis
Valvular heart defects is a great clinical problem in developing countries because of high incidence of rheumatic heart diseases and in Western countries because of high incidence of degenerative valve diseases [1]. Such patients often require the implantation of a valve prosthesis — mechanical or biological. These two types of the valves differ from each other in service life and thrombogenicity. Unlike the mechanical prosthesis, the biological one is less thrombogenic on the one side and has a shorter life duration on the other side.
The patients undergone the operation for the replacement of the heart valves by the mechanical prostheses are at the risk of developing thromboembolic complications, the most formidable of which are thrombosis of valve prostheses and disabling strokes, which, in turn, can lead to the death of a patient [2][3]. Thromboembolism can be caused by the following: the occurrence of turbulent blood flow and blood stasis, which are created by the implanted valve itself, as well as high thrombogenicity of the mechanical prosthesis material [4]. It should be noted that the place of prosthetics plays an important role in the assessment of thrombogenic risk factors: unlike the aortic valve replacement, the mitral valve replacement has a higher risk of thromboembolic complications; this, in turn, leads to higher values of the target international normalized ratio (INR) and therefore, to an increased risk of bleeding [5]. Thus, antithrombotic therapy is necessary for everyone in this group of patients in the postoperative period. The antithrombotic drugs include indirect anticoagulants — vitamin K antagonists (VKA) (warfarin, acenocoumarol, phenprocoumarol) and antiplatelet agents (aspirin, dipyridamole and clopidogrel) [6][7]. It is important to note that the effect of each of these drugs on clotting factors and on the degree of platelet aggregation predisposes to bleeding.
The major meta-analysis including 13 studies with 4122 patients was published in 2013 by Cochrane Collaboration. The research showed that compared to VKA alone, the addition of antiplatelet agent (aspirin/dipyridamole) not only reduced the risk of thromboembolic complications but also increased the risk of bleeding [8]. However, this meta-analysis had a number of significant limitations: first of all, the most studies were done before 1990, and the implanted prostheses had high rates of thrombogenicity; secondly, the research investigated relatively small sample size (the majority <200 people); thirdly, the studies contained many patients with concomitant ischemic heart disease that can explain the benefits of additional prescription of antiplatelet drugs. It should be noted that in 6 studies of 13 in the meta-analysis, dipyridamole was used as an antiplatelet agent, which is currently rarely used in clinical practice.
Thus, the optimal strategy for combined antithrombotic therapy in patients with mechanical prosthetic heart valves is still an open question.
The purpose of our systematic review and metaanalysis is to evaluate the efficiency and safety of combined oral anticoagulant therapy (VKA) and aspirin antiplatelet therapy in comparison with VKA monotherapy in patients after mechanical heart valve replacement.
Material and methods
Search for publications and selection of studies.
The information retrieval algorithm was developed in accordance with the reporting requirements and regulations for systematic reviews and meta-analyses (PRISMA) [10][11]. These recommendations help to describe the study so that it can be evaluated by editors, reviewers, readers, as well as other researchers engaged in meta-analysis. The literature was searched in databases MEDLINE/PubMed (www.ncbi.nlm.nih.gov/pubmed) and Google Scholar.
To search data in PubMed we used the following keywords: “warfarin” OR “vitamin k antagonists” AND “mechanical heart-valve” AND “aspirin” AND “thromboembolism”. We also used a manual search in the links from the certain review articles, metaanalyses and consensus statements. To search data in Google Scholar we used the following: mechanical valve replacement, valvular heart disease, anticoagulation, vitamin K antagonists, warfarin, antiplatelet, aspirin, prosthesis, thromboembolism, bleeding, stroke, efficiency, safety. The selection of suitable studies for inclusion into this systematic review and meta-analysis was carried out by following: the two authors studied abstracts and full-text articles for compliance with the inclusion criteria independently from each other.
The last data search for inclusion into this analysis was done on 05.02.2022.
Criteria for inclusion/exclusion. The criteria for inclusion into the systematic review followed by meta-analysis were: only randomized clinical trials were included; the studies with access to full texts; all participants were adult (18 years old and above); the research which studied the group of patients after mechanical heart valve replacement, where was the comparison between addition of aspirin to VKA (warfarin) and VKA (warfarin) monotherapy. Apart from that, a prerequisite for the inclusion of the publications in the meta-analysis was the presentation of clinical outcome data, such as thromboembolic events, major bleeding and mortality. The minimum follow-up period in the study was 6 months. The articles in languages other than English, descriptions of individual cases, preclinical studies, reviews and expert opinions, as well as studies whose results are published only in the form of abstracts, were excluded from the meta-analysis.
Besides, to describe the basic characteristics, the following data were taken from each study: mean follow-up period, average age of patients and gender distribution, target INR or prothrombin time ratio, aspirin dosage, type and location of the prosthetic valve.
Assessment of methodological quality. The systematic error (Risk of bias) was assessed in accordance with the Cochrane criteria for the assessment methodological quality of randomized clinical trials (RoB 2 tool) [12]. All discrepancies were eliminated by discussion between the authors of the work.
Statistical analysis. All types of statistical analysis were carried out using the program RevMan 51. The main results are depicted graphically as forest plot or blobbogram. Statistical heterogeneity was evaluated using Pearson’s chi-squared criterion, as well as the heterogeneity index I2. The interpretation of the statistical heterogeneity assessment according to the index I2 was carried out by following the recommendations of the Cochrane Collaboration, under which I2=0-40% corresponds to weak heterogeneity; 30-60% — moderate heterogeneity; 50-90% — substantial heterogeneity; 75-100% — high heterogeneity. Also, statistical heterogeneity was assessed by p-value defined by criterion ?2, where p<0,1 — presence of statistically significant heterogeneity, and р?0,1 — absence of statistically significant heterogeneity. The effect was measured mainly using the odds ratio (OR) with 95% confidence interval (CI). The effect was considered statistically significant at p<0,05. For OR calculation to assess the effect we used a fixed effects model. The possibility of systematic errors associated with predominant publication of positive research results was analyzed by means of visual assessment of the funnel plot.
Results
Literature search results. The total number of the publications found in databases PubMed and Google Scholar using the keywords was 2377. The number of publications after excluding the duplicates was 2298. After analyzing the headers and abstracts, only 56 publications matching the set goal remained. The most frequent reasons for the exclusion of the articles were the set goal mismatch and the absence of specified data; we also excluded the review articles, discussions, abstracts and reports. After full-text screening, 11 articles remained. However, two of them did not give the data of the number of outcomes in the groups [13][14], and one study [15] did not meet the criteria for a randomized trial because it was cohort, and therefore, these studies were also excluded from our analysis. Thus, 8 studies were finally selected for our review; the process of the selecting relevant studies is shown in Figure 1.
General characteristics of study. For the present systematic review and meta-analysis, 8 randomized clinical trials were selected [9][16-22]. The articles included in the systematic review and meta-analysis were published in the period from 1976 to 2014. The total number of patients was 4082. The average age of the patients was 50.8 years old, men — 2484 (60,9%). The average follow-up period was 1,75 years (Tables 1, 2).
In all included studies, the antiplatelet agent was aspirin at doses of 500 mg/day [16], 1000 mg/day [17], 200 mg/day [20], 100 mg/day [18][19][21], 75-100 mg/day [9][22].
A number of studies preceded the appearance and widespread use of the INR value, and the target level of anticoagulation was estimated by an increase of prothrombin time 1,8-2,3 times as long as in normal [16] or 10% increase of thrombo-test relatively to normal [17]. It should be noted that in two studies, the target INR was 1,8 to 2,5 [9][22]; in three studies, the high level of the target INR was from 3,0-3,5 to 4,5 [18][19][21]; and in one study, the target INR values were 2,5 to 3,5 [20].
Endpoints and adverse outcomes. One of the criteria for inclusion into the systematic review was the presence of the endpoint reports — thromboembolic events, bleeding and lethality. Of the main outcomes, the criteria for thromboembolic events or arterial thromboembolism were clearly defined for each trial. In the most trials, the definitions of thromboembolic complications were similar and included the following conditions: ischemic stroke or transient ischemic attack, other systemic thromboembolism confirmed by ultrasound and/or surgery. In one trial [20], non-obstructive thrombosis of mechanical prostheses and transient ischemic attack were considered as minor embolic events. However, for our meta-analysis, such outcomes were classified as major thromboembolic events.
The data of serious hemorrhagic complications in the included studies were less unambiguous. In the Meschengieser`s study 1997 [19], a major hemorrhagic event was defined as bleeding required hemotransfusion, hospitalization or if it was the cause of death. In Turpie`s study 1993 [18], major hemorrhage was defined as obvious bleeding associated with a drop in hemoglobin levels by >20 g/l, the need for transfusion of >2 units of blood, or any intracranial, intraocular, intra-articular or retroperitoneal hemorrhage. In LIWACAP study 2007 [21], the concept of major hemorrhage included the following: intracranial hemorrhage confirmed by computed tomography; retroperitoneal hemorrhage, also confirmed by computed tomography; intraocular hemorrhage, led to blindness; intra-articular hemorrhage; bleeding associated with a drop in hemoglobin levels by >20 g/l or the need for transfusion of >2 units of blood or required surgical intervention. In two studies [16, 17], there were no classification of hemorrhages into major and minor therefore, in our meta-analysis, we considered any cases of intracerebral and gastrointestinal hemorrhage, and also the episodes of hemoptysis, as significant hemorrhagic events. In Laffort`s study 2000 [20], hemorrhages were also classified into major and minor: major hemorrhage was defined as bleeding associated with a sudden drop in hemoglobin levels by >20 g/l or bleeding required transfusion of >2 units of blood or required surgical intervention as well as any intracranial hemorrhage. In two studies [9][22], there were no definition of the concept of major and minor hemorrhages but were given the data of the bleeding localization therefore, for our meta-analysis, we considered significant bleeding as cerebral hemorrhage.
The data of lethality were available for all the trials and included general lethality. In one study only (LIWACAP 2007) [21], the concept “lethality” implied sudden cardiac death (death that occurred within an hour after the manifestation of symptoms and was not provoked by a non-vascular cause); the study did not report of any other cases of death.
Risk of systematic error in the included studies. The funnel plots for thromboembolic events, mortality and massive hemorrhages did not show any signs of publication systematic error (Figures 2, 3 and 4).
Thromboembolic events. The total number of thromboembolic events in the VKA with aspirin group was 49 (2,4% of 2037 patients), while in the VKA monotherapy group — 105 (5,1% of 2045 patients). The addition of aspirin to VKA led to a statistically significant decrease in thromboembolic complications. Compared with taking VKA only, the combination of VKA and aspirin significantly reduced the risk of thromboembolic complications by 2,1 times (OR: 0,47; 95% CI: 0,33-0,67; р<0,0001). The heterogeneity test was insignificant (р=0,63, I2=0%) (Figure 5).
Lethality. The total number of lethal cases in the VKA + aspirin group was 36 (1,8% of 2037 patients), while in the VKA monotherapy group — 105 (3,0% of 2045 patients). The combined analysis of lethal events showed that the total mortality was 1,7 times statistically significant lower in the aspirin + VKA group versus VKA monotherapy (OR: 0,58; 95% CI: 0,38-0,88; p=0,01). The heterogeneity test was insignificant (p=0,22, I2=26%) (Figure 6).
Major hemorrhages. The total number of the cases of bleeding occurrence in the VKA + aspirin group was 78 (3,8% of 2037 patients), while in the VKA monotherapy group — 58 (2,8% of 2045 patients). The meta-analysis showed that the frequency of the major hemorrhages increased with combined therapy VKA + aspirin versus VKA monotherapy but these differences did not reach statistical significance (OR: 1,41; 95% CI: 0,99-2,01; р=0,06). The heterogeneity test was insignificant (р=0,14, I2=36%) (Figure 7).
The table 3 shows the summarized results reflected the occurrence of the main clinical outcomes in the patients after mechanical heart valve replacement in comparison groups between VKA + aspirin therapy and VKA monotherapy.
Analysis of subgroups depending on the intensity of anticoagulant therapy. As it is known, the increase of INR greater than the target values against the background of taking VKA increases the risk of bleeding. Our meta-analysis includes two studies [19][21], in which for the VKA monotherapy group, the higher target values of INR were defined (from 3,0-3,5 to 4,5), while in the aspirin + VKA group, the target values of INR were from 2,5 to 3,5. The total number of the cases of bleeding occurrence in the aspirin + VKA group was 10 (2,8% of 352 patients), while in the VKA monotherapy group — 13 (3,7% of 349 patients). The meta-analysis showed that the risk of major hemorrhages did not differ between the groups (OR: 0,76; 95% CI: 0,33-1,74; р=0,51) (Figure 8).
We also analyzed the influence of these two studies [19][21] on the endpoints of death and thromboembolic complications. The total number of thromboembolic events in the VKA + aspirin group was 7 (2,0% of 352 patients), while in the VKA monotherapy group — 8 (2,3% of 349 patients), lethal outcomes — 10 (2,8% of 352 patients) and 21 (6,0% of 349 patients), respectively. The metaanalysis showed that the risk of thromboembolic events did not differ statistically significant in the VKA + aspirin group versus the VKA monotherapy group (OR: 0,85; 95% CI: 0,31-2,30; р=0,75); at the same time, the frequency of the lethal outcomes statistically significant decreased (ОR: 0,45; 95% CI: 0,21-0,96; р=0,04) (Figures 9, 10).
Therefore, for the verification of the possible impact on the overall results of the meta-analysis, the studies with high values of target INR (Meschengieser 1997 [19] and LIWACAP 2007 [21]) were excluded in the general analysis.
The total number of the cases of major hemorrhages in VKA + aspirin group was 68 (4,0% of 1685 patients), while in VKA monotherapy group — 45 (2,7% of 1696 patients). After excluding the above studies, the meta-analysis showed that the risk of bleeding statistically significant increased with the addition of aspirin to VKA versus VKA monotherapy (OR: 1,63; 95% CI: 1,1-2,42; р=0,02) (Figure 11).
We also analyzed the influence of the exclusion of these studies on the endpoints of death and thromboembolic complications. The total number of thromboembolic events in the VKA + aspirin group was 42 (2,5% of 1685 patients), while in the VKA monotherapy group — 97 (5,7% of 1696 patients), lethal outcomes — 26 (1,5% of 1685 patients) and 40 (2,4% of 1696 patients), respectively. The metaanalysis showed that the risk of thromboembolic events statistically significant decreased in the VKA + aspirin group versus VKA monotherapy (OR: 0,42; 95% CI: 0,29-0,61; р<0,00001); at the same time, the frequency of the lethal outcomes did not differ statistically significant between the groups (OR: 0,65; 95% CI: 0,39-1,08; р=0,09) (Figures 12, 13).
Analysis of subgroups depending on the monitoring method of the anticoagulant therapy effectiveness
As already noted, until 1990, there was no standardized assessment of the effectiveness of VKA therapy after mechanical heart valve replacement. In our meta-analysis, we analyzed the subgroups which were formed in accordance with the era of research — before and after 1990 (the year of the beginning of widespread use of standardized INR). Before 1990, there were just two studies — Altman [16] and Dale [17], where 270 patients were included. The six studies were published after 1990 (Turpie [18], Meschengieser [19], Laffort [20], LIWACAP [21], Dong [22], Wang [9]); the total number of the included patients were 3812. The risk of thromboembolic events statistically significant decreased in both subgroups (before and after 1990) in the VKA + aspirin group versus VKA monotherapy (before 1990: OR: 0,20; 95% CI: 0,07-0,54; р=0,002; after 1990: OR: 0,53; 95% CI: 0,37-0,77; р=0,0008) (Figure 14). In our meta-analysis, the number of the lethal outcomes in the subgroup before 1990 does not differ significantly in the VKA + aspirin group versus VKA monotherapy (OR: 0,49; 95% CI: 0,14-1,67; р=0,25); on the contrary, in the subgroup after 1990 the risk of lethal outcomes statistically significant decreased in the VKA + aspirin group (OR: 0,59; 95% CI: 0,38-0,92; р=0,02) (Figure 15). The meta-analysis showed that in the subgroup before 1990, the risk of major hemorrhage occurrence was statistically significant higher in the VKA + aspirin group versus VKA monotherapy (OR: 2,52; 95% CI: 1,05-6,04; р=0,04), while in the subgroup after 1990 there were no significant differences (OR: 1,25; 95% CI: 0,84-1,84; р=0,27) (Figure 16). Probably, the obtained results regarding the increase of the risk of major hemorrhage occurrence in the dual therapy group are associated not only with the absence of standardized assessment of the VKA therapy effectiveness but also with the use of aspirin in large doses when added to VKA: in the Altman`s study [16] the dose was 500 mg/day, in Dale`s study [17] — 1000 mg/day.

Figure 1. Flowchart of the selection of the studies included in the review.
Table 1
Characteristics of the studies included in the systematic review. Location and type of mechanical prosthesis

Abbreviations: AV — aortic valve, MV — mitral valve, n/d — no data, N — the number of the patients included in the study.
Table 2
Main characteristics of the patients included in the study

Abbreviations: VKA — vitamin К antagonist, INR — international normalized ratio, n/d — no data, PT — prothrombin time.
Table 3
Comparison between combination of VKA and aspirin and VKA monotherapy

Abbreviations: OR — odds ratio, CI — confidence interval.

Figure 2. Funnel plot: aspirin + VKA versus VKA monotherapy. Thromboembolic risk.

Figure 3. Funnel plot: aspirin + VKA versus VKA monotherapy. Lethal cases.

Figure 4. Funnel plot: aspirin + VKA versus VKA monotherapy. Hemorrhages.

Figure 5. Forest plot OR (logarithmic scale) for thromboembolic risks depending on addition of aspirin to VKA compared with VKA monotherapy.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 6. Forest plot OR (logarithmic scale) for lethal outcomes depending on addition of aspirin to VKA compared with VKA monotherapy.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 7. Forest plot OR (logarithmic scale) for major hemorrhages depending on addition of aspirin to VKA compared with VKA monotherapy.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.
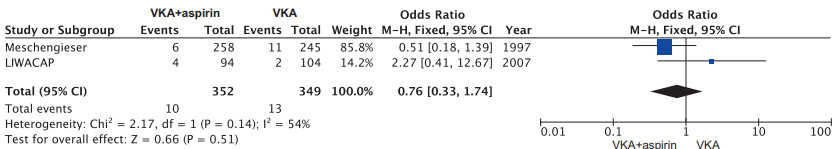
Figure 8. Forest plot OR (logarithmic scale) for major hemorrhages in the studies of high-intensity warfarin therapy.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 9. Forest plot OR (logarithmic scale) for thromboembolic events in the studies of high-intensity warfarin therapy.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 10. Forest plot OR (logarithmic scale) for lethal outcomes in the studies of high-intensity warfarin therapy.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 11. Forest plot OR (logarithmic scale) for major hemorrhages depending on addition of aspirin to VKA compared with VKA monotherapy. The Meschengieser 1997 and LIWACAP 2007 studies are excluded.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 12. Forest plot OR (logarithmic scale) for thromboembolic risks depending on addition of aspirin to VKA compared with VKA monotherapy. The Meschengieser 1997 and LIWACAP 2007 studies are excluded.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 13. Forest plot OR (logarithmic scale) for lethal outcomes depending on addition of aspirin to VKA compared with VKA monotherapy. The Meschengieser 1997 and LIWACAP 2007 studies are excluded.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 14. Forest plot OR (logarithmic scale) for thromboembolic risks depending on addition of aspirin to VKA compared with VKA monotherapy. The analysis of subgroups that include the studies performed before and after 1990.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 15. Forest plot OR (logarithmic scale) for lethal outcomes depending on addition of aspirin to VKA compared with VKA monotherapy. The analysis of subgroups that include the studies performed before and after 1990.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.

Figure 16. Forest plot OR (logarithmic scale) for major hemorrhagic events depending on addition of aspirin to VKA compared with VKA monotherapy. The analysis of subgroups that include the studies performed before and after 1990.
Note: The center of each line represents the OR for each study, and the ends of the horizontal lines represent 95% CI. A continuous vertical line represents an OR equal to 1.
Abbreviations: VKA — vitamin K antagonist, CI — confidence interval, OR — odds ratio.
Discussion
The 2020 guideline of American College of Cardiology (АСС)/American Heart Association (AHA) [23] on managing patients with heart failure reported of the decrease of the recommendation class and the level of evidence for adding aspirin to VKA for patients after mechanical heart valve replacement (class 2b, level B-R). It should be recalled that the previous АСС/AHA guideline on managing patients with heart failure (2017) recommended to add aspirin at doses 75-100 mg to VKA therapy for all patients with mechanical heart valve prostheses (1А) [24]. It should be noted that this recommendation was based mainly on the results of two small studies (Turpie, Meschengieser), performed in 1993 and 1997, respectively [18][19]. The Turpie`s study 1993 included 370 patients, the majority of which had ischemic heart disease therefore, the prescription of aspirin in addition to warfarin naturally led to the decline in mortality from cardiovascular causes unlike with warfarin monotherapy, while the risk of major hemorrhage in the groups was comparable. However, when studying the structure of the occurred hemorrhages, it was obvious that such a severe complication as intracranial hemorrhage occurred in 8 patients who took aspirin together with VKA, and in just 3 patients who took VKA only. The Meschengieser`s study 1997 [19] which included 503 patients, compared VKA monotherapy against the background of the maintenance of high INR values (3,5-4,5) and the addition of aspirin to warfarin against the background of the INR values from 2,5 to 3,5. It is notable that the frequency of thromboembolic events in VKA monotherapy group compared with the aspirin + VKA group was the same (2,8% and 2,7%, respectively); similarly, the frequency of major hemorrhages in the groups did not differ (4,5% and 2,3%, respectively). As in the previous study, when investigating the structure of the occurred hemorrhages, it was found that in the warfarin monotherapy group against the background of high INR values, 3 intracranial hemorrhages occurred, whereas none in the combination therapy group.
The decrease of the class and the level of evidence for adding aspirin to VKA for patients after mechanical heart valve replacement in the recommendations of АСС/AHA 2020 is based on the results of a major systematic review and meta-analysis published in 2013 by Cochrane Collaboration (Cochrane Database of Systematic Reviews). The analysis included 13 studies with total number of patients 4122 in the period from 1971 to 2011. Compared with VKA monotherapy, the addition of antiplatelet agent (apirin/dipyridamol) was associated with statistically significant decrease of the risk or thromboembolic events by 2,3 times (OR: 0,43; 95% CI: 0,32-0,59; p<0,00001) and total mortality by 1,7 times (OR: 0,57; 95% CI: 0,42-0,78; р=0,0004). However, at the same time, the risk of major hemorrhage also statistically significant increased (OR: 1,58; 95% CI: 1,14-2,18; р=0,006), that, apparently, was the reason for the decrease in the class and level of evidence of the recommendations [8].
It should be noted that the quality of the most studies included in this systematic review, was low that perhaps reflected the era when some of them were performed (1970s and 1980s i.e. before the appearance of widespread use of standardized INR) [25].
In our meta-analysis, we investigated the addition of aspirin to VKA as the most common antiplatelet agent used in clinical practice. The obtained results regarding to statistically significant decrease of the frequency of thromboembolic events convince us in undoubted benefit of the addition of aspirin to VKA. However, the safety profile issue of the combination of aspirin and VKA remains controversial. For instance, in our work, the combined analysis of all the eight studies showed the absence of statistical significance in the frequency of the major hemorrhage occurrence against the background of aspirin + VKA therapy compared with VKA monotherapy, whereas the Cochrane Collaboration meta-analysis 2013 showed statistically significant increase in the frequency of hemorrhagic events in the dual therapy group. Probably, the results we obtained are associated with addition of Wang`s study 2014 [9] included quite a large number of patients (N=1016) into the meta-analysis. Apart from that, when added aspirin to VKA, the low dosage was used (75-100 mg/day), and the target INR was within the limits from 1,8 to 2,5. It is also important to note that during heart valve replacement surgery, the modern mechanical prostheses with low thrombogenic profile were used, that allowed to maintain low values of INR and thereby, to decrease the risk of bleeding. For instance, in the Wang`s study 2014, the frequency of thromboembolic complications in the group with combination of OAC and low-dose aspirin was 2,2% compared with 4,3% when use OAC only (OR: 0,48; 95% CI: 0,23-1,01). The frequency of major hemorrhagic events was comparable, and in both groups it was 0,6% (OR: 0,99; 95% CI: 0,20-4,94).
Conclusion
The addition of aspirin to VKA versus VKA monotherapy showed statistically significant risk reduction of systemic embolism and death in patients with mechanical heart valve prostheses, in the absence of statistically significant differences in the frequency of major hemorrhages. The results we obtained require further verification and conducting large-scale research with use of the modern mechanical heart valve prostheses with low thrombogenic profile and possible maintenance of lower target INR values when taking VKA together with aspirin to assess the efficiency and safety of this combination of drugs.
Relationships and Activities: none.
1. Review Manager (RevMan) [Computer program]. Version 5.4.1, The Cochrane Collaboration. 2020.
