Takayasu’s arteritis in a patient with suspected acute coronary syndrome — a literature review and a case report
Nonspecific aortoarteritis (Takayasu’s arteritis (TA), pulseless disease) is an autoimmune disease characterized by destructive-proliferative segmental aortitis and subaortic panarteritis of elastic-fiber-rich arteries with possible coronary and pulmonary artery involvement [1][2][3].
The true prevalence of the disease is unknown. TA is registered everywhere, but the number of cases in the population varies depending on the continent, race, sex and age [4]. The highest prevalence (40 cases per million population) is recorded in Japan, while the lowest (0,9 per million) one in the United States. Modern epidemiological studies register an increasingly widespread distribution of TA in Europe (from 0,4 to 1,5 cases per 1 million) [4]. There is evidence that in Russia its prevalence reaches 2,6 people per 1 million population [3]. The disease is most typical for young females with onset at the age of 15-35 years (according to various data, the womanto-man ratio is from 2:1 in Western countries to 10:1 in Eastern ones) [1][2].
The specific cause of the disease has not been established — the role of viruses, infections, in particular, mycobacterium tuberculosis, is being discussed. There is also information about the genetic predisposition. This theory is supported by the fact that one third of patients with TA has HLA-B52 allele [1][2].
TA is characterized by multiple segmental damage to the aorta and aortic branches, followed by development of stenoses, occlusions, and aneurysms. First of all, the inflammatory process is localized in the media and adventitia, with further spread to the perivascular tissue. The intima involvement is of a secondary reactive-hyperplastic nature [3]. TA can be suspected after an objective examination of a patient, given the very specific localization of pathological process. As a rule, these are carotid and subclavian artery stenosis, the prevalence of which reaches 96% [1].
Depending on lesion localization, 6 types of TA are distinguished [2]:
- Type I — aortic arch and its branches;
- Type IIa — ascending aorta, aortic arch and its branches;
- Type IIb — ascending aorta, aortic arch and its branches, thoracic descending aorta;
- Type III — thoracic descending aorta, abdominal aorta, and/or renal arteries;
- Type IV — abdominal aorta and/or renal arteries;
- Type V — combined features of types IIb and IV.
When the coronary and/or pulmonary arteries are involved, C (+) or P (+) is added to the type of disease. The coronary and pulmonary artery involvement occurs in approximately equal numbers of cases, 5-20% and 7-18%, respectively. Right coronary artery involvement was the most common [1][2].
On examination, the most characteristic sign is weak pulse in one or both of the radial arteries up to complete absence, due to the typical localizations described above [2][3]. Attention is also drawn to the difference between systolic blood pressure (BP) on the right and left hands by more than 10 mm Hg. During auscultation, murmur in the projection of affected vessel can be detected [2].
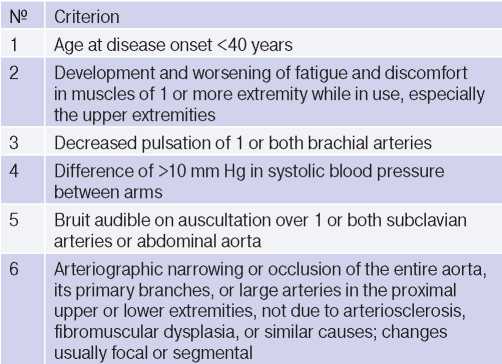
At the moment, in clinical practice, the 1990 American College of Rheumatology criteria (Table 1) are used for diagnosis [2][3]. The presence of three or more of any criteria in a patient makes it possible to diagnose TA with a specificity of 98% and a sensitivity of 91% [2].
Table 1
1990 American College of Rheumatology criteria for the classification of Takayasu arteritis [3]
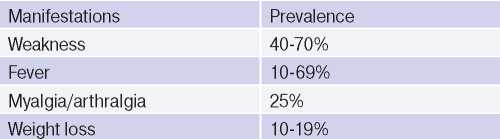
The clinical performance, syndromes and symptoms described in the disease depend on its stage. The initial stage of TA is characterized by systemic inflammation in the form of low-grade fever, weakness, weight loss, myalgia and arthralgia (Table 2). As a rule, from 0,5 to 2 years is required to make diagnosis from the symptoms’ onset. In most cases, when a patient turns to a physician, there is already late stage of disease [1][2].
Table 2
General clinical manifestations in AT [1]
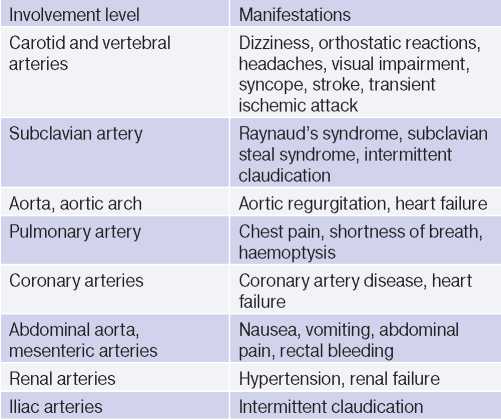
In the late TA stage, the leading symptoms are prolapse and/or dysfunctions of organs due to stenoses, occlusions and thrombosis of the arteries supplying them. A wide variety of symptoms is due to the multilevel involvement of the aorta and its branches (Table 3). In one patient, signs of active and inactive TA phases may be present at the same time, given that the pathology is recurrent in nature [1][2][3].
Table 3
Possible clinical manifestations [1]
It is worth mentioning the existing clinical classification of TA by K. Ishikawa (1978). This classification reflects the natural clinical course in the absence of therapy with the most serious complications, such as secondary hypertension, retinopathy, aortic regurgitation and aneurysms (Table 4) [1].
Table 4
Ishikawa clinical classification of Takayasu arteritis [1]

The main death causes in TA are stroke, heart failure, acute types of coronary artery disease, and renal failure. Given this, this classification is useful in determining the prognosis in this group of patients. In patients with or without one mild complication, vascular events are absent in 97% of cases within 5 years, whereas in patients with one or more severe complications, adverse vascular events develop in 40,3% of patients within the same time period [1].
For many years, angiography has been the gold standard in TA diagnosis, but given that this method is invasive, it has a higher risk of complications compared to non-invasive techniques [2]. The main reason for angiography at the moment are indications for percutaneous transluminal angioplasty and/ or stenting [5].
In patients with suspected TA, magnetic resonance imaging (MRI) should be used as the first diagnostic test to assess the lesion. Magnetic resonance angiography and contrast-enhanced MRI make it possible to assess the degree of vascular stenosis and to identify tissue and morphological changes in the arteries. It has been proven that MRI has 100% sensitivity and specificity for TA diagnosis. But, given the cost of this diagnostic method, low availability, other imaging techniques are at the first lines of diagnostics [2].
An imaging technique that is widely used in clinical practice is multislice computed tomography (MSCT). Contrast-enhanced MSCT is the fundamental method in the description of TA anatomy, which allows visualizing the thickening of the aorta and its branches, as well as stenosis degree. This technique has high sensitivity and specificity in TA diagnosis [1][2].
Duplex ultrasound plays an important role in visualization of the inflammatory process. Its main advantages are its availability, simplicity, cost, the ability to measure the carotid intima-media thickness [2].
The above methods are recommended for dynamic monitoring of structural vascular damage and assessing the therapy effectiveness [2].
18F-fluorodeoxyglucose positron emission tomography is one of the TA diagnosis methods, which currently makes it possible to assess the disease activity and involvement. The accumulation of radiopharmaceuticals in areas with active inflammation demonstrates the degree and morphology of arterial damage [1].
TA is treated with glucocorticosteroid (GCS) therapy in order to achieve and maintain remission. With GCS monotherapy, remission can be achieved in 40-60% of cases. When patients are resistant to GCS therapy, cytostatic and/or biological agents are added to the treatment. The need for combination therapy of TA varies from 40 to 84% of cases, but only 40% of patients in this group can achieve stable remission [1].
Azathioprine and methotrexate are the most widely used cytostatics in TA treatment [1]. Increasingly popular are biological agents — anti-IL-6 receptor monoclonal antibodies (tocilizumab) and anti-tumour necrosis factor-? antibodies (infliximab, certolizumab, etanercept). As mentioned earlier, this group of drugs is used in case of ineffectiveness of conventional immunosuppressive therapy [2].
However, the disease continues to progress and remains resistant to all possible options for drug therapy in 20% of patients with TA [1].
In addition, TA patients are diagnosed with an increased content of thrombin-antithrombin III complex, fibrinopeptide A and D-dimers. Considering the hypercoagulation with increased BP, the risk of vascular events in target organs significantly increases. Thus, antiplatelet therapy is necessary as the primary prevention of TA complications [1].
As mentioned above, it is recommended to use invasive interventions to correct stenosing and occlusive lesions of the arteries. Recently, percutaneous transluminal angioplasty with or without stenting has become the most popular [5]. The frequency of surgical interventions for TA varies from 12 to 70%. The indications should include manifestations of coronary artery disease, complicated renal artery stenosis, stroke, and aneurysms. In such situations, carrying out revascularizing procedures significantly increases survival and reduces the death risk [1].
It is worth mentioning the TA register launched in 2016 in East China. Given such a rare diagnosis, the standardization of the management of such patients is often difficult. Thus, researchers are trying to recruit as many TA patients as possible by creating a TA cohort for more professional and standardized patient management.
Case report
Forty-eight-year-old male patient was delivered by an ambulance team with non-ST-elevation acute coronary syndrome. Upon admission, he complains of pressing pain in chest with physical exertion (brisk walking, climbing 1-2 floors), shortness of breath and increased pain in the right chest half with a deep breath, low exercise tolerance, peripheral oedema.
Collection of history revealed a BP increase for ~5 years up to 200/100 mm Hg. The current deterioration was within 1,5 months, characterised by shortness of breath, pressing pain in chest with intensification during exertion, puffiness, low exercise tolerance. He noted an increase in body temperature up to 370C, a progressive weight loss of 25 kg in 6 months. He did not seek medical help. On the eve of admission, anginal pain became more frequent, intensified, dyspnoea increased. He turned to the local outpatient clinic. After electrocardiography (ECG), hospitalization was recommended to the cardiology department.
At the time of admission, the condition was of moderate severity, clear consciousness, skin of normal colour, moist, warm. Muffled heart sounds, regular heart rate of 80 beats per min. A significant difference in BP was found: 170/90 mm Hg on the right hand and 130/80 mm Hg on the left one. Lung auscultation revealed weakened vesicular sounds in the lower areas. The abdomen was soft and painless. The liver protrudes 1,5 cm below the costal margin. There was no costovertebral angle tenderness on both sides.
At admission, ECG showed incomplete left bundle branch block, low-amplitude R and deep negative T waves in I, AVL, V3-V6 leads (Figure 1).
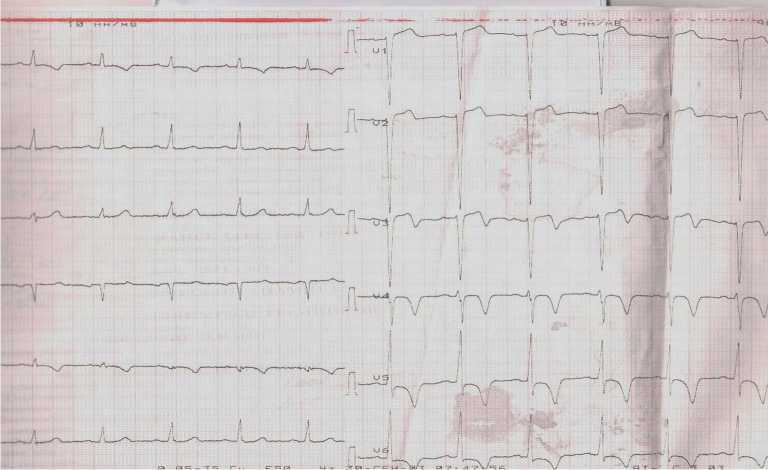
Figure 1. ECG of the patient upon admission to the hospital.
There were no significant changes in high-sensitivity troponin at admission and three hours later (0,008/0,014 ng/ml). There were following findings: white blood cells — 19,38?109/L, C-reactive protein — 62,8 mg/L (inflammatory markers); total platelet count — 642,0 10*3/ml, (thrombocytosis); red blood cells — 4,52?1012/L; hemoglobin — 110,0 g/L (mild anemia); creatinine — 131,4 mmol/L, glomerular filtration rate — 53,8 ml/min/1,73 m2 (reduced renal function); total cholesterol — 5,24 mmol/L, low-density lipoproteins — 3,23 mmol/L, high-density lipoproteins — 1,17 mmol/L (dyslipidemia).
According to echocardiography, the cardiac cavities were not dilated. Left ventricular (LV) ejection fraction was 59%. There were akinetic areas in apex, lower third of interventricular septum, anterior wall, and apical-lateral segment. Heart valves were normal. Left ventricular hypertrophy (interventricular septum — 13 mm, LV posterior wall — 15 mm) was revealed. The pericardium: above the right ventricle — 16 mm, the right atrium — 16 mm, along the LV posterior wall — 15 mm. Pleural cavity expansion: on the right — 26 mm, on the left — no.
According to the data obtained, the diagnosis of acute coronary syndrome raised doubts. Therefore, it was decided to conduct additional diagnostic tests.
Taking into account the increased body temperature, progressive weight loss, it was decided to perform chest, abdominal and pelvic MSCT. The MSCT revealed pronounced diffuse thickening of thoracic and abdominal aortic walls with infiltration of the paraaortic tissue. This process extended to left subclavian and both renal artery orifices, narrowing the lumen to 80% (Figure 2). There were no thrombotic masses in pulmonary artery and its branches. There were no abnormalities in the lung parenchyma. Also, worth noting is the thickening and infiltration of the perinephric tissue. The excretory renal function was sharply reduced.
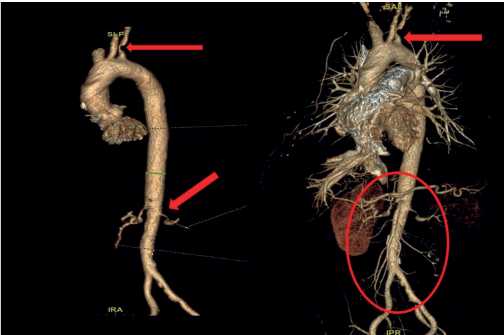
Figure 2. 3D reconstruction of the thoracic and abdominal aorta and their branches (arrows mark the lesions).
The next stage of diagnosis was coronary angiography to assess the degree of coronary involvement, followed by decision on the need for myocardial revascularization. There was a right type of cardiac blood supply. The left coronary artery was with irregular contours. The left anterior descending artery was irregular contours and extended stenosis of 50-75% from the orifice to proximal third of the 2nd segment (Figure 3). Intermediate artery: occlusion from the orifice, distal areas are filled through intracoronary collaterals (Figure 3). The circumflex artery was with irregular contours, stenosis of 40% from the orifice, extended stenosis of 30% in the distal third of the 1st segment. The right coronary artery was with irregular contours and without hemodynamically significant stenoses (Figure 4). It was decided to perform stenting of affected artery segment using the Resolute Integrity (Medtronic) 3,0×30 mm stent (Figure 5).
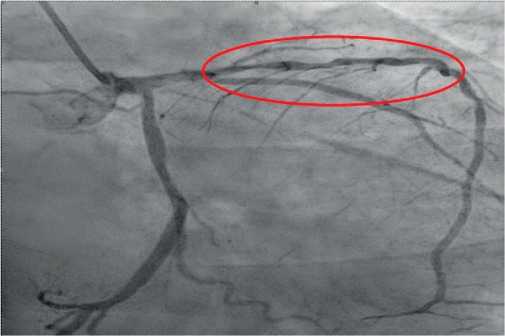
Figure 3. Extended stenosis of 50-75% from the orifice to proximal third of the 2nd segment (RAO 300-Caudal 300).
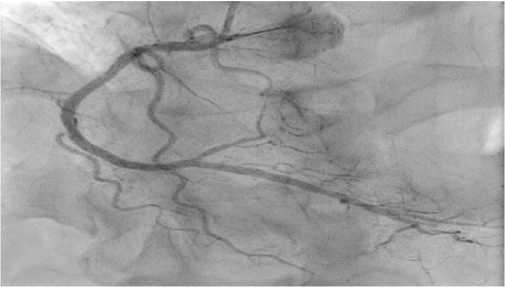
Figure 4. No abnormalities in the right coronary artery (RAO 300-Caudal 300).
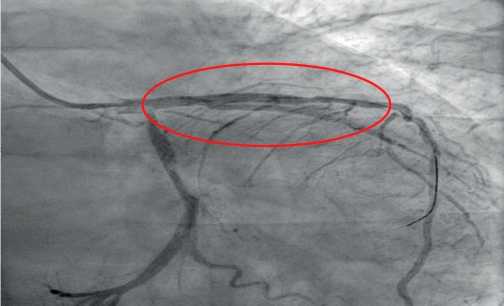
Figure 5. Left anterior descending artery after stenting surgery.
Subsequently, the patient underwent duplex ultrasound of brachiocephalic arteries: intima-media thickness — 1,3 mm and 1,2 mm on the left and right sides, respectively. There were following stenoses: left and right common carotid artery up to 55% and 60%, respectively; left and right internal carotid artery up to 28% and 30%, respectively; right external carotid artery up to 35%. Vertebral-subclavian steal syndrome.
It is worth noting the laboratory test dynamics during the follow-up period (Table 5).
Table 5
Laboratory test dynamics during the follow-up period
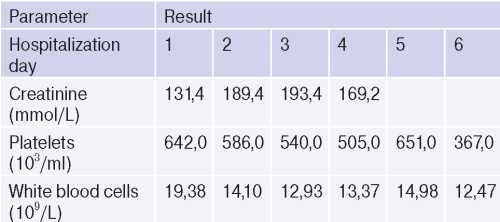
The rest of blood test parameters were within the reference values and without negative dynamics. Blood tests for HIV, RW, hepatitis were negative.
According to repeated echocardiography, resolving hydropericardium and hydrothorax were shown.
Thus, based on imaging and laboratory research methods indicating an inflammatory process in the walls of arteries of multiple localization, the patient was diagnosed as follows:
Non-specific type V C+ TA, with involvement of thoracic, abdominal aorta, coronary arteries (left anterior descending artery up to 75%), subclavian arteries, brachiocephalic arteries (left and right common carotid artery up to 55% and 60%, respectively; left and right internal carotid artery up to 28% and 30%, respectively; right external carotid artery up to 35%, left subclavian artery), renal arteries (up to 80%).
Unstable high-risk class 2 angina pectoris. Coronary angiography, left anterior descending artery stenting. Secondary hypertension. Left vertebral subclavian steal syndrome. Resolving moderate hydropericardium. Resolving bilateral mild hydrothorax. Stage 2А NYHA class 2 heart failure. During hospitalization, the patient received the following treatment: acetylsalicylic acid, clopidogrel, sartans, beta-blockers, calcium channel blockers, loop diuretics, verospiron, statins. After treatment, the patient noted a significant improvement
As discussed above, the cornerstone of TA therapy is immunosuppressive therapy, in particular — GCSs. Considering the need to take dual antiplatelet therapy, a positive history of gastrointestinal diseases (increased risk of bleeding), poorly controlled hypertension, the appointment of GCS therapy was postponed until a rheumatologist was consulted. Unfortunately, the period of hospitalization of our patient coincided with the introduction of COVID19 restrictions and reprofiling of most departments in large hospitals in Samara, which did not allow a prompt consultation with a rheumatologist.
At discharge, the patient was given extended recommendations on the necessary follow-up examination and drug therapy.
The patient was invited for a consultation with a cardiologist 6 months after discharge from the hospital. During this period, the patient was not hospitalized in other hospitals. He noted lower limb oedema while taking amlodipine, as a result of which he independently cancelled it. Objective examination findings: BP on the right and left hands was 160/80 and 110/70 mm Hg, respectively. Complete blood count and biochemical blood test were without abnormalities. ECG was without negative dynamics compared to the early one. Taking into account the irrational therapy and not reaching the target BP levels, the treatment was adjusted. The patient was consulted by a rheumatologist and an additional examination was recommended: a biochemical blood test (rheumatoid factor, C-reactive protein, antidouble stranded DNA antibodies (anti-dsDNA), antinuclear factor, antinuclear antibodies (ana), ANCA), angiography of aorta and its large branches. According to available results, anti-dsDNA, ANA, ANCA IgG were within normal limits. Anti-inflammatory therapy was not prescribed. After the remaining examinations, a second consultation with a rheumatologist was recommended.
Thus, the clinical picture of acute coronary syndrome in some cases can be caused not only by coronary atherosclerosis, but also be a consequence of occlusive-stenotic lesions of the aorta and its branches due to connective tissue diseases, which require differential diagnosis and alteration of management and therapy strategies with taking into account the identified conditions.
