Novel biological markers for the diagnosis and prediction of mortality risk in patients with pulmonary embolism
One of the forms of venous thromboembolism (VTE) — pulmonary embolism (PE) — ranks third in the structure of causes of death among all cardiovascular diseases, being behind the myocardial infarction and stroke. From 39 to 115 cases of PE per 100 thousand people are registered in the world, annually [1]. That is why the timely and the earliest possible diagnosis of VTE is having a special importance, which will help to improve both shortterm and long-term prognosis of patients.
Diagnosis of PE at the first contact with medical personnel may be difficult due to the presence of non-specific signs and symptoms, such as cough, shortness of breath, tachypnea, hemoptysis, chest pain, which can be observed in a number of other diseases [2]. In addition to the assessment of right ventricular (RV) function by transthoracic echocardiography (EchoCG) and computed tomographic angiography (CT angiography), have been also used the following biological markers — D-dimer, N-terminal pro-brain natriuretic peptide (NT-proBNP) and troponin I [3].
For example, the D-dimer is used in clinical practice to diagnose acute forms of VTE and predict the risk of their recurrences. The D-dimer is the product of the splitting of a fibrin clot by plasmin. At the same time, it has a high sensitivity — a negative result allows to confidently rule out PE in patients with a low and medium probability of it, with an accuracy of up to 97%. However, the D-dimer has a low specificity in the diagnosis of PE, resulting from its increase in many physiological and pathological conditions (pregnancy, cancer, inflammatory processes, and others) [4].
NT-proBNP is a peptide hormone that is produced by cardiomyocytes of the heart ventricles. When the RV is overloaded with pressure, the myocardium is overstretched and damaged, which leads to the release of this hormone and troponin I. The level of natriuretic peptides and troponin I in blood plasma shows the severity of RV dysfunction in acute PE [5, 6].
Heart troponin I, like NT-proBNP, is an important predictor of inhospital or 30-day mortality [7]. In a meta-analysis by Bajaj A, et al., elevated troponin I levels were significantly associated with an increased risk of short-term mortality (odds ratio (OR), 4,80; confidence interval (CI), 95%, 3,257,08), PE-related mortality (OR, 3,80; CI 95%, 2,74-5,27), and serious adverse events (OR, 3,65; CI, 95% 2,41-5,53) [8].
Considering the insufficient specificity of the above-mentioned laboratory parameters, there is an urgent need to search for new biomarkers that can improve the quality of detection and stratification of VTE, including PE. The diagnostic and prognostic test for the verification of PE should be accurate, safe, easily accessible and not expensive, as well as reproducible and non-invasive [9, 10]. However, at present, none of the diagnostic tests used meets all these criteria, so it is reasonable to further search for appropriate markers. Our review presents the currently available literature data on the latest laboratory indicators that characterize the dysfunction of the RV, which develops because of PE, and have an evidence base for the stratification of death risk in this category of patients.
Heart-type cytoplasmic fatty acid-binding protein (H-FABP)
H-FABP is a cytoplasmic protein with a mass of 15 kDa that is highly expressed in cells with active lipid metabolism. It facilitates the intracellular transport of long-chain fatty acids and mainly located in the myocardium (~0,5 mg/g), small amounts of it are presented in the brain and skeletal muscles. H-FABP enters the bloodstream 2 hours after myocardial injury, reaches concentration in 6-8 hours, and returns to normal within 24-36 hours after previous myocardial ischemia. According to a study by Kaczynskaya A, et al., cytoplasmic protein exceeded cardiac troponin I, NT-proBNP and myoglobin in prognosis of 30-day mortality associated with PE. In this study, the levels of myoglobin, cardiac troponin I, NT-proBNP, and H-FABP were evaluated in 77 patients with a confirmed PE (mean age, 65,3±16 years). Hazard ratio analysis showed that plasma concentrations of H-FABP, myoglobin, cardiac troponin I, and NT-proBNP correlated with 30-day mortality. According to the presented multivariate analysis, the predictive ability of H-FABP measured at admission was even higher than for myoglobin, cardiac troponin I, and NT-proBNP [11].
Similar data were obtained in the study of Bajaj A, et al., who published the result of a meta-analysis of 15 large-scale studies that revealed a direct correlation between increased H-FABP levels and the risk of 30-day complications, such as the need for thrombolytic therapy, endotracheal intubation, catecholamine support for hypotension, recurrence of PE and mortality. The sensitivity and specificity of H-FABP were 71% and 74% for predicting 30-day complications, and 90% and 70% for predicting 30-day mortality, respectively [12].
Another meta-analysis by Dellas C, et al. presents a conclusion based on the examination of 1680 patients with PE, for whom the levels of this protein were defined. The authors concluded that H-FABP >6 ng/ml was associated with an unfavorable shortterm outcome (OR, 17,7; 95% CI, 6,0-51,9) and all-cause mortality (OR, 32,9; 95% CI, 8,8-123,2) [13]. As a result, cytoplasmic protein is mentioned as a biological marker of myocardial damage in the recommendations of the European Society of Cardiology (ESC) for the diagnosis and conduction of patients with PE in the 2014 and 2019 versions [3, 14]. Thus, H-FABP is an early marker of myocardial damage and, therefore, with the help of H-FABP, additional prognostic information about acute PE can be obtained.
Growth differentiation factor-15 (GDF-15)
Growth differentiation factor-15, also known as macrophage inhibitory cytokine 1, is a protein from the superfamily of transforming growth factor-beta that is synthesized in the myocardium after ischemic and reperfusion injury, as well as during pressure overload of the RV. Circulating levels of GDF- 15 have prognostic significance for the patients with acute coronary syndrome and heart failure [15]. Lankeit M, et al. presented the results of a investigation that evaluated the significance of GDF-15 in predicting the outcomes of PE. The Cox regression model established a nearly 3-time increase in the risk of death for the patients with elevated levels of GDF-15. Using a multivariate logistic regression, laboratory parameters (troponin T and NT-proBNP), and EchoCG data, GDF-15 appeared as an independent predictor of 30-day complications (p=0,033). It also showed higher levels of sensitivity and specificity (Figure 1, 2) — the area under the curve (AUC) for GDF-15 was 0,84 (95% CI, 0,76-0,90) compared to 0,72 (95% CI, 0,63-0,80) for cardiac troponin T and 0,65 (95% CI, 0,56-0,73) for NT-proBNP. Thus, the GDF- 15 in combination with troponin I, NT-proBNP and EchoCG with the signs of RV dysfunction can increase the reliability of the prognosis assessment. Basing on the results of this study, baseline levels of GDF-15 were identified as independent predictors of long-term mortality (p<0,001). Basing on the above-mentioned factors, the authors conclude that GDF-15 can be considered as a new promising biomarker for stratifying the death risk due to PE [16]. However, to include GDF-15 in the list of examinations of patients with PE, further research is required.
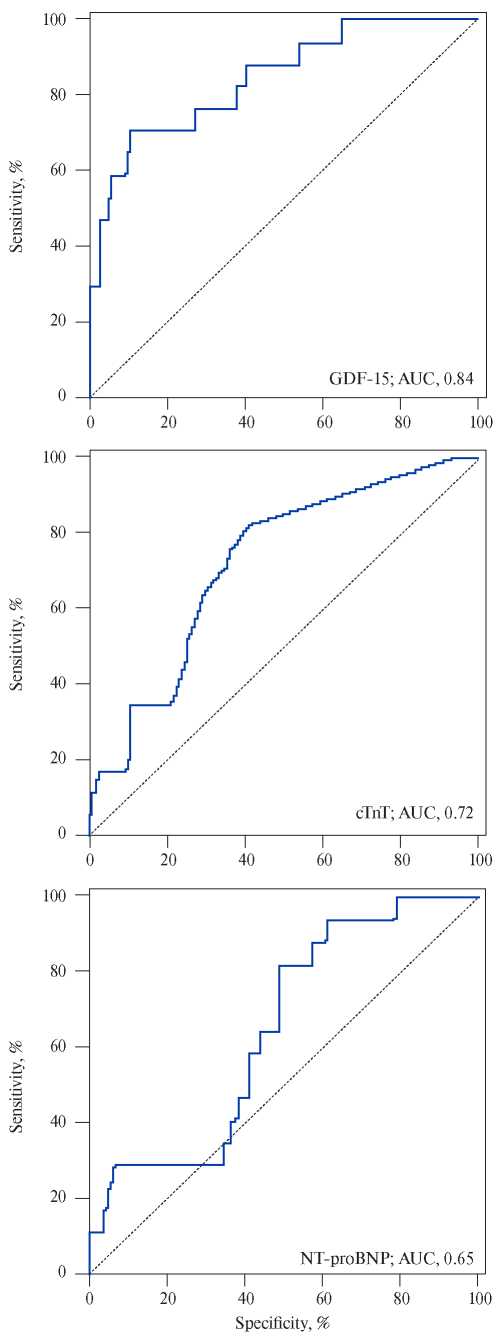
Figure 1. Prognostic sensitivity and specificity of growth dif – ferentiation factor (GDF-15), cardiac troponin T (cTnT), and N-terminal pro-brain natriuretic peptide (NT-proBNP).
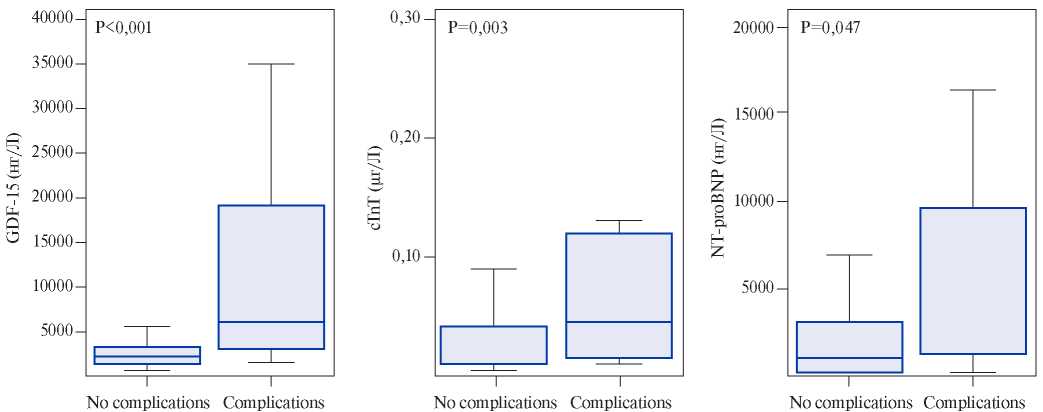
Figure 2. Baseline biomarker levels in patients with severe complications in contrast to patients with an uncomplicated 30-day outcome. GDF-15, cTnT, and NT-proBNP levels are expressed as box (25th percentile, median and 75th percentile) and whisker (10 and 90 percentiles).
Copeptin
Copeptin is the C-terminal part of provasopressin and is a glycosylated polypeptide that consists of 39 amino acids and contains a leucine-saturated main segment. The significance of copeptin has been studied for acute conditions such as pneumonia, sepsis, heart failure, lower respiratory tract infections, gastrointestinal diseases, and ischemic stroke [17]. Nickel NP, et al. found out that copeptin levels were elevated in patients with pulmonary hypertension (PH) [18]. Serum copeptin levels were evaluated in a retrospective cohort of 92 untreated PH patients and in a second prospective cohort of
15 PH patients treated immediately after diagnosis. A comparison of the results showed that the levels of circulating copeptin were increased for untreated patients with PH compared to patients in the control group who received therapy (20,1 pmol/L vs 5,1 pmol/L; p=0,001). Copeptin levels did not correlate with hemodynamic parameters, but decreased after the start of PH therapy (p=0,001). Elevated copeptin levels were associated with the decline of survival level (p<0,001) and proved to be an independent predictor of mortality (OR, 1,4; 95% CI, 1,1-2,0; p=0,02). The result of the study suggests that this polypeptide may be a fairly significant prognostic marker in the stratification of the death risk for patients with acute PE.
In a small study conducted by Kalkan AK, et al. (n=90), patients were divided into 2 groups, depending on the result of CT angiography: with PE (+) (n=47) and without PE (-) (n=43). Copeptin levels were higher in the PE (+) group compared to the PE (-) group: 7,76±4,4 vs 3,81±1,34 ng/ dl; p<0,001, respectively. Copeptin levels were significantly correlated with NT-proBNP (r=0,434, p<0,001), D-dimer (r=0,315, p=0,003), and troponin I (r=0,30, p=0,004). An inverse correlation was also found with arterial blood oxygen saturation (r=-0,533, p<0,001) [19]. Thus, the data obtained so far shows that copeptin can be considered as a promising biomarker that will be used as an addition to D-dimer, troponin I, and NT-proBNP in order to improve the accuracy of PE diagnosis [20].
Indicators of complete blood count
A complete blood count is one of the most common primary diagnostic procedures. This is a publicly available, widely used, and low-cost study. However, a number of parameters of complete blood count, as a rule, are not considered by clinicians in the aspect of diagnosing and predicting the risk of VTE.
Red blood cell distribution width (RDW)
Red blood cell distribution width is part of a complete blood count analysis, which reflects the range of changes in the volume of red blood cells. The main biomolecular mechanism of RDW-PE association is not fully understood, but it is assumed that elevated RDW levels correlate with acute inflammatory markers and blood viscosity indicators. It is believed that such indicators as RDW and the number of red blood cells are important factors contributing to the formation of such prothrombotic conditions as PE and deep vein thrombosis (DVT) [21].
In 2019, a systematic review was published by Hammons L, et al., combining 12 retrospective cohort studies that demonstrated that high levels of RDW are associated with an increased risk of acute PE, severity, and increased mortality in patients with PE. However, the comparison of current studies is limited due to the lack of common approaches to determining the upper limit of RDW (each study uses a different reference value of RDW), the formation of a sample, a wide range of exclusion criteria and the inclusion of various methods used for the diagnosis of PE. Despite the above limitations, the authors of a systematic review showed that RDW as a marker of PE has the right to exist [22]. However, considering the presented data, the significance of RDW in this area requires further study.
Mean Platelet Volume (MPV)
MPV reflects the mean platelet volume. There is evidence that MPV is an important variable, since larger platelets have a higher thrombotic potential [23]. MPV is not used as an independent marker in the diagnosis of PE. However, the determination of this indicator may be useful for the initial assessment of PE risk. Elevated MPV has been recognized as an independent risk factor for various clinical conditions associated with hypercoagulation. Gulcan M, et al. (2012) showed that MPV was significantly increased in patients with DVT compared to the control group (8,6±0,8 vs 7,7±0,9 fl, respectively; p<0,001) [24]. Given that PE is a complication of DVT in 50% of cases, MPV may also be directly related to PE [25].
Talay F, et al. published a retrospective study that included patients with suspected PE. As a result of the subsequent sample, 150 patients with confirmed PE and 165 patients without PE were examined [26]. MPV was significantly higher for patients with PE in the conditions of intensive care unit than in the control group (9,42±1,22 fl vs 8,04±0,89 fl, p<0,0001). The area under the curve for patients with a clinically suspected PE, according to the ROC analysis, was 0,634 (95% CI, 0,5960,702, p=0,023) (Figure 3). The result of this study demonstrated a moderate relationship between the MPV value and PE, if the first was determined at the time of admission of the patient.
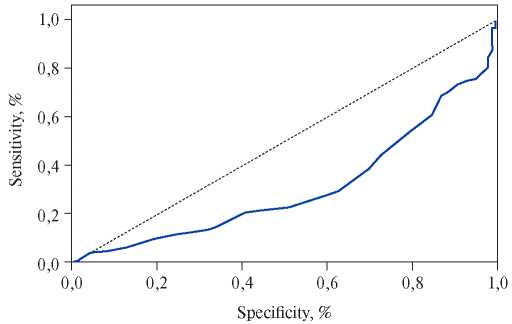
Figure 3. ROC analysis of the role of MPV in patients with a clinically suspected PE.
In a prospective cohort study of Ghaffari S, et al., the various parameters of complete blood count were also studied, including the MPV. According to the ROC analysis, the sensitivity and specificity of MPV with a cut-off point of 9,85 fl in predicting inhospital mortality was 81% and 50%, respectively. This parameter had lower efficacy for longterm mortality (AUC, 0,54; 95% CI, 0,47-0,61) compared to 30-day mortality. Thus, this marker has demonstrated its significance in determining inhospital all-cause deaths [27]. Perhaps in the future, MPV can prove itself to be a simple and early marker for predicting the death risk for patients with PE, which will become part of the standard protocol for the conducting of patients with VTE.
Platelet-to-lymphocyte ratio (PLR) and neutrophilto-lymphocyte ratio (NLR)
The pathophysiological response of white blood cells to a stress is usually an activation of the sympathetic nervous system and the release of cortisol, as well as an increase in the number of neutrophils, which is associated with a decrease in the number of lymphocytes. This leads to the migration of neutrophils to the affected area. PLR and NLR, as surrogate markers of inflammation, can be used to study the relationship between thrombosis and inflammation during VTE. One of the advantages of PLR and NLR is that they combine information about primary hemostasis and inflammation.
The case-control study by Artoni A, et al., which included 486 patients with VTE, demonstrated that patients with high PLR or NLR did not have an increased risk of VTE complications (OR, 0,89; 95% CI, 0,46-1,76; OR, 0,69; 95% CI, 0,34-1,39, respectively) or cerebral venous thrombosis (OR, 1,65, 95% CI, 0,68-4,00; OR, 0,39. 95% CI, 0,091,72, respectively). The authors concluded that there is no association between PLR and NLR values above the acceptable norm and an increased risk of venous thrombosis [28]. However, the data presented are not sufficient for the widespread use of PLR and NLR as markers in the diagnosis of PE, so further larger studies in this area are needed.
Surfactant protein A (SP-A) and D (SP-D)
Surfactant is a lipoprotein complex that consists of several phospholipids, neutral lipids (90%), and specific proteins. Protein components, half of which consist of two groups of surfactant-associated proteins: hydrophobic — B (0,7%), C (0,4%), and hydrophilic — A (5,3%) and D (0,6%), make up approximately 10% of the lung surfactant [29]. Among these proteins, SP-A is the most common lung surfactant protein [30, 31]. SP-A is also necessary for the structure of tubular myelin. In a study made by Liu CP, et al. the SP-A level for 32 rats was 1,00±0,00 (control group), 0,44±0,18 (after 24 h), 0,44±0,33 (after 1 week) and 0,52±0,32 (after 2 weeks), respectively (p<0,05), which is conditioned by the developing hypoxia with PE [32]. Thus, the level of SP-A protein significantly decreased in acute PE.
Reduced SP-A expression in the lungs during embolism may be caused by a limited number of type II cells and/or reduced SP-A expression of the remaining intact cells. At the moment, however, it is difficult to draw conclusions about the significance of SP-A in the diagnosis and prediction of PE risk in humans.
In addition to SP-A, other proteins have been studied, in particular SP-D. Kati C, et al. presented a small study in which 3 groups of patients were identified: patients with diagnosed non-massive PE, submassive PE, and a control group that included healthy people. SP-D levels were determined by immunofluorescence analysis. There were no significant differences in the parameters of this protein between the control group and the group with non-massive PE, but its level was increased in patients with submassive PE [33]. Given the small number of patients (n=60), further studies with a large number of cases, including patients with massive PE, are required.
Thus, qualitative and quantitative changes in the lung surfactant, in particular its protein fractions, are potential markers of lung damage and can be prognostic indicators in patients with PE. In the future, it advisable to pay attention to the presented biomarkers and conduct additional research in this area.
Lipocalin-type prostaglandin D synthase (L-PGDS)
L-PGDS can be considered as a protein with a dual function: first, it acts as an enzyme in the production of prostaglandin D2, and, secondly, as an extracellular transporter, due to its lipophilic nature. This marker was first isolated in human cerebrospinal fluid in 1961. MicroRNA of L-PGDS has been found in myocardial cells, in atrial and ventricular endocardial cells, in coronary arteries, in smooth muscle cells, and even in arteriosclerotic plaques. During the last years, the role of L-PGDS has been more frequently studied in the evaluation of kidney function as an alternative to creatinine, as well as as a possible biomarker of cardiovascular diseases.
The prospective study, presented by Mutlu H, et al., involved 90 patients who were admitted to the emergency department with PE, confirmed by CT angiography, as well as 40 healthy volunteers without any diseases. L-PGDS levels were measured in venous blood. For all patients, the risk of death within 30 days was calculated according to the PESI index. As a result, there were significant differences between the levels of L-PGDS in patients with PE and the control group (p=0,024), and the 1-month mortality rate for patients diagnosed with PE was 20% (n=18). The limit value for L-PGDS obtained using the ROC curve analysis for 1-month mortality was 815,26 ng/ml (sensitivity, 83,33%; specificity, 79,17%; AUC, 0,851; OR, 95% CI, 0,760-0,917; p<0,001). Based on this threshold, the logistic regression analysis showed that an increase in L-PGDS simultaneously with an increase in the PESI class (r=0,512, p<0,001) is an independent indicator of death during the first month. The obtained result allows us to consider L-PGDS for predicting the risk of mortality for patients with PE [34].
Circulating and tissue microRNA
MicroRNAs are endogenously expressed RNA molecules with a length of 18-22 nucleotides that suppress gene expression at the post-transcriptional level by binding to the three prime untranslated region of the target mRNA. MicroRNAs are involved in almost all biological processes — in cell proliferation, apoptosis, and cell differentiation. It is known that microRNAs play a role in the cardiovascular pathophysiology, including hemostatic disorders [35]. There is evidence that microRNAs are secreted from cells into human biological fluids in both passive and active ways. Such microRNAs are called circulating microRNAs. The change in the expression profile of certain circulating microRNAs reflects the physiological and pathological conditions of cells in which microRNAs are modified and secreted into human biological fluids, such as blood, urine, cerebrospinal fluid, saliva, etc. Circulating microRNAs can be found in various forms — enclosed in exosomes or bound to Ago2 proteins. Due to these forms of transport, the circulating microRNAs are stable and protected from degradation by ribonucleases. Therefore, circulating microRNAs are considered as new potential biomarkers, interesting in many diseases, including VTE.
Xiang Q, et al. analyzed 12 studies in the field of VTE diagnostics that studied microRNAs and presented the following conclusions. The most frequently studied microRNA was miR-134, and the combined results of 12 studies on the predictive ability of this microRNA with a 95% CI showed a sensitivity of 0,82 (0,69-0,91) and a specificity of 0,83 (0,68-0,92). The average AUC value for the ROC curves was 0,89 (0,86-0,92). For other microRNAs, AUC values >0,8, were considered as potential diagnostic indicators. These microRNAs included miR-1233, miR-145, miR-483-3p, miR- 582, miR-532, and miR-195 [36]. Thus, this systematic review allows to pay attention to the presented microRNAs in the field of predicting the development of PE in a particular patient.
A study made by Kessler T, et al., which included a small number of patients (n=30), demonstrated that the profile of circulating microRNA-1233 allows to distinguish PE from ST-segment elevation myocardial infarction. MicroRNA-1233 differentiated patients with PE from patients with myocardial infarction and healthy people with
sensitivity of 90 and 90% and specificity of 100 and 92% (AUC, 0,95, p<0,001 and AUC 0,91, p<0,001, respectively) [37]. In the study of Lui T, et al., which also included a small number of patients (n=90), microRNA-221 was determined, the appearance of which correlated with the level of NT-proBNP, troponin I, and D-dimer [38]. Therefore, some microRNAs are possible diagnostic and prognostic markers in patients with PE. However, further research in this area is required to determine the specific microRNAs for PE.
Apolipoproteins СI, CII, CII, and E
Apolipoproteins are the protein components of lipoprotein molecules which characterize the blood lipid spectrum. An increase in their concentration is associated with an increased risk of arterial thrombosis [39]. In addition, apolipoproteins can potentially be important in assessing the risk and prognosis of VTE, since an increase in the number of apolipoproteins affects hemostasis and leads to hypercoagulation.
In the study of Orsi FA, et al., a total of 127 patients with VTE and 299 patients without a history of VTE were included. The selection of patients was carried out randomly. The level of apolipoproteins was determined for all patients. The results showed that increased levels of all measured apolipoproteins were associated with higher levels of vitamin K-dependent blood coagulation factors (FII, FVII, FIX, FX, FXI), natural anticoagulants (protein C, protein S, antithrombin), and clot lysis time. In addition, an increase in coagulation factor VIII and von Willebrand factor levels correlated with an increase in apoC-III and apoE levels. Age-and sex- adjusted OR of apolipoproteins E, C-III, CII, and CI to the risk of venous thrombosis were 1,21 (95% CI, 0,98-1,49), 1,19 (95% CI, 0,99-1,44), 1,24 (95% CI, 0,95-1,61) and 1,06 (95% CI, 0,87-1,30) [40]. The authors conclude that the levels of apolipoproteins C-I, C-II, C-III, and E are associated with a variety of blood apoC-III coagulation factors and physiological anticoagulants. At the same time, in the work performed by Van Schouwenburg IM, et al., the lipid profile parameters were not associated with VTE risk [41]. Therefore, this issue remains controversial at the moment, and further research is required to clarify it.
Thus, according to current clinical guidelines, some biological markers (D-dimer, cardiac troponin I, NT-proBNP) are important tools for diagnosing and predicting the risk of death for patients with PE. However, their use has a number of limitations and disadvantages, as a result of which, various biological molecules are currently being studied for the diagnosis of PE. Some of them GDF-15, microRNA, MPV, L-PGDS, SP-A, and SP-D – showed good predictive abilities. At the same time, many of the biomarkers listed in this review are not used in normal clinical practice due to insufficient evidence base. Nevertheless, the results presented on this topic provide the foundation for larger studies and the search for new diagnostic markers.
