Prediction of sinus rhythm maintenance after radiofrequency ablation in patients with atrial fibrillation using speckle tracking echocardiography and dynamics of left atrial structural and functional parameters
Atrial fibrillation (AF) is the most common arhythmia associated with complications such as heart failure, left ventricular (LV) dysfunction, and impaired quality of life [1]. Due to cardioembolic events, AF is associated with an increased risk of stroke and death [1]. Recent studies have shown that the provoking triggers of AF are factors such as obesity, smoking, alcohol, and hypertension [1]. However, the solution of some issues in the diagnosis and treatment of patients with AF remains ambiguous. As it is known, AF occurrence depends on left atrial (LA) size. A number of studies [2][3] report a directly proportional relationship between the risk of AF onset/recurrence with LA increase degree. However, recent studies indicated that some patients with paroxysmal or persistent AF have no LA remodeling. Then the main risk factor for recurrent AF is myocardial fibrosis with mechanical impairment of LA. As some authors have shown, an indirect sign of LA myocardial fibrosis was impaired wall compliance in the reservoir phase [4]. The most adequate method for assessing LA mechanics was speckle-tracking echocardiography [3]. However, the threshold values of strain (S)/ strain rate (SR) depending on LA phase are not standardized and are not known to all doctors involved in echocardiography.
As is known, in patients with AF, resistant to antiarrhythmic therapy, the method of choice is pulmonary vein radiofrequency ablation (RFA) [1][5]. According to retrospective studies, the use of this method reduces the risk of ischemic stroke and mortality in the world [1][5]. RFA efficacy has also been proven in the treatment of elderly patients with paroxysmal AF complicated by acute left ventricular failure [6]. In general, after RFA, the quality of life of patients significantly improves [1][7]. Despite this, AF recurrences are common [2][4][5][8][9]. Over the past decade, there has been an active search for reliable markers of RFA efficacy using various diagnostic methods, including using speckle-tracking echocardiography [8-11]. The interpretation of routine echocardiographic parameters of LA in patients referred for catheter ablation is still controversial. New guidelines on diagnosis and treatment of AF patients recommends to determine not only the size, but also the LA function [1]. The use of speckle-tracking echocardiography will probably allow the cardiology team to develop an individual approach to the management/treatment of AF patient.
Hypothesis: it is assumed that effectiveness of RFA depends on the initial morphological and functional LA parameters in AF patients.
The aim was to evaluate the dynamics of LA volume (LAV), S during the reservoir phase and SR in patients with paroxysmal and persistent AF, scheduled for RFA, as well as to compare the predictive value of S and SR as a marker of maintaining sinus rhythm.
Material and methods
The study protocol was approved by the Ethics Committee of the Bashkir State Medical University. All patients signed written informed consent. In the Bashkir State Medical University Clinic, 35 patients aged 40-75 years old with complaints of rapid uneven heartbeat were examined, scheduled for electrophysiological study (EPS)-RFA (Figure 1). There were following inclusion criteria: documented paroxysmal/persistent AF in a patient without prior rheumatic mitral valve disease and myocardial infarction, as well as the patient’s consent to EPS-RFA. All participants underwent following examinations: resting electrocardiography (ECG), 24-hour Holter ECG monitoring (HM), echocardiography (transthoracic and transesophageal), coronary angiography. As a result, 3 patients had moderate mitral stenosis, 2 — severe interventricular septal myocardium (IVS) hypertrophy (IVS ?1,2 cm in women; IVS ?1,3 cm in men). In 5 patients, coronary angiography revealed hemodynamically relevant stenosis (?50%). Two patients previously underwent endovascular interventions for AF (1st patient — balloon cryoablation; 2nd — catheter RFA). At the first stage, 12 of the above patients were excluded from. Thus, the criteria for excluding patients with AF were mitral valve disease, previous AF ablation, prior cardiac surgery, myocardial infarction, NYHA class II-IV heart failure, thyroid disease.

Figure 1. Distribution of AF patients scheduled for RFA by groups.
Abbreviations: CAS — coronary artery stenosis LVH — left ventricular hypertrophy, RFA — radiofrequency ablation, MS — mitral stenosis, AF — atrial fibrillation.
At the second stage, 12 months after EPS-RFA, 2 patients from remote villages of the Republic of Bashkortostan could not come for reexaminations. In addition, cancer was identified in 2 patients 1 year after EPS-RFA that required chemotherapy, which was the reason for their exclusion.
A control group was formed of 20 subjects without arrhythmias, comparable in age and sex: 12 men (60%) and 8 women (40%) aged 59,0±11,2 years.
As a result, we analyzed 19 patients who underwent pulmonary vein ablation, aged 62,32±10,76 years, among whom there were 11 men (58%) and 8 women (42%) included in the prospective study. Despite the initial therapy with class IC, II or III antiarrhythmics, they still had AF: paroxysmal AF — 33% (n=6); persistent AF — 67% (n=13). All patients after RFA underwent ECG and HM immediately after RFA and after 12 months, as well as when the patient had symptoms suggestive of arrhythmia recurrence, which was defined as a registered AF episode >30 sec according to ECG or HM [1].
Using two-dimensional echocardiography in all patients, no more than 2 weeks before RFA and a year after this procedure, the structural and functional left heart parameters were assessed: left ventricular (LV) end diastolic dimension (EDD) (cm), LV EDD index (cm/m2), LV mass (g), LV mass index (g/m2), LV ejection fraction (%), LA volume (LAV, ml), LAV index (LAVI, ml/m2). For the statistical sample, the maximum LAV in the reservoir phase was taken into account. The maximum LAV was obtained by tracing the LA endocardial wall immediately before mitral valve opening, excluding the appendage and pulmonary veins. Using 20 disc method, a score was automatically obtained. The upper normal limit for 2D LA volume was 34 ml/m2, regardless of sex differences [12].
Data on LA longitudinal S using the EPIQ 7 ultrasound software (Philips, USA) was obtained during registration from QRS beginning (first peak of positive LA S in the reservoir phase). For this, using a standard two-dimensional ECG synchronized grayscale echocardiography, at a frame rate ?80 per sec (after exhalation while holding the breath), an image was obtained from the 4- and 2-chamber apical view with a clear LA endocardium visualization. In the post-processing mode, LA border was traced below the mitral annulus level by 10 mm. In addition, the peak positive LA S (%) in the reservoir phase in four- (4C) and two-chamber (2C) views was assessed as the average value from all LA walls. According to the meta-analysis, the lower normal limit for LA reservoir phase was considered average strain value equal to 39% [11][13]. LA SR (s-1) in the reservoir phase was monitored using automatic graphs, and the average SR of all LA walls in 4C and 2C views was taken into account. No unambiguous threshold values for SR in LA reservoir phase have been established. In this regard, we were guided by the data from foreign studies, which indicated that reservoir-phase SR in healthy individuals was 4,1 s-1 [9].
RFA was performed on an empty stomach in stable patients after discontinuation of antiarrhythmic therapy for a period of fivefold drug’s half-life. Under local anesthesia with 0,25% lidocaine solution (6,0 ml) by puncturing the right femoral vein with 2 Biosense Webster Preface Multiporse 8 Fr 62 cm introducers, transpesophageal echocardiography guided transseptal puncture (St. Jude Medical BRK 1) was carried out. Intracardiac echocardiography imaged the thin area of septum. It was punctured guided by X-ray and intracardiac echocardiography. Through the first introducer, a Biosense Webster LASSO 2515 Nav diagnostic catheter was carried for placement in the area of pulmonary vein orifices. A Biosense Webster Navi Star ThermoCool F-type ablation catheter (Diamond Bar, USA) was passed through the second introducer. Intracardiac electrograms were recorded using the Prucka Cardio Lab electrophysiological system (General Electric Marquette, Inc., Milwaukee, WI, USA). Threedimensional LA mapping was performed using the CARTO 3 system (Biosense Webster, Diamond Bar, California, USA) in the FAM mode using diagnostic and ablation catheters. Then, pulmonary vein orifices was isolated with an ablation catheter, which was confirmed by pacing from the ablation and diagnostic electrodes. After RFA, all patients continued 8-week antiarrhythmic therapy and 12-week anticoagulant therapy (then, therapy was continued in women with CHA2DS2-VASc score ?2, men — ?1) [1].
Statistical processing was carried out using Statistica 10 and IBM SPSS Statistics 26 software. Quantitative data are presented as mean (M) and standard deviation (SD). The significance of differences (p) between the groups was assessed using parametric tests (normally distributed variables) — two-sample Student’s t-test. Comparison of nonnormally distributed variables was carried out using the Mann-Whitney U-test. To check the effectiveness of S and SR, Receiver Operating Characteristic analysis (ROC) was used. The area under the curve (AUC) was determined and cutoff points associated with AF recurrence were established.
Results
To analyze the results among AF patients 12 months after RFA, two groups of patients were selected: group 1 — patients without recurrent AF after RFA, group 2 — patients with recurrent AF after RFA. Group 1 included 12 of 19 initial patients (63%), who maintained sinus rhythm according to ECG and HM within 12 months after RFA. Group 2 included 7 out of 19 initial patients (37%) with recurrent AF within 12 months after RFA. There was no age difference between the patients of two groups: group 1 — 66,50±11,50 years; group 2 — 56,00±9,35 years old (p=0,11).
Echocardiography revealed that LAV in group 1 patients became less 12 months after RFA (47,0±12,1 ml) than the initial LAV (56,0±12,6 ml) (p=0,008). The same changes of LAVI in group 1 was revealed (Figure 2): initially, 28,0±7,8 ml/m2 vs 12 months after RFA, 22,6±8,3 ml/m2 (p=0,02). In group 2 patients with ineffective RFA, there was no decrease in either LAV or LAVI 12 months after RFA: initial LAV, 52,0±23,2 ml vs LAV after RFA, 54,0±12,1 ml (p=1,0); initial LAVI, 25,1±13,6 ml/m2 vs LAVI after RFA, 30,9±7,6 ml/m2 (p=0,3).

Figure 2. LAVI dynamics in patients without AF recurrence 12 months after RFA.
Abbreviations: LAVI — left atrial volume index, RFA — radiofrequency ablation, AF — atrial fibrillation.
Analysis of LA S in group 1 patients with effective RFA did not reveal changes in longitudinal strain in the 4C- and 2C-view: 4C-S initially, 32,2±11,1% vs 4C-S after RFA, 30,3±9,6% (p=0,287); 2C-S initially, 26,1±9,8% vs after RFA, 28,9±9,1% (p=0,82).
In group 2 patients with ineffective RFA, LA S in 4C- and 2C-views did not significantly change depending on the RFA: 4C-S initially, 21,4±0,6% vs 4C-S after RFA, 17,4±6,2% (p=0,12); 2C-S initially, 16,2±3,2% and 2C-S after RFA, 16,5±6,8% (p=1,0).
When comparing the initial LAV (Figure 3) and LAVI (Figure 4), no differences were found between the 1st and 2nd groups (p=0,78 and p=0,85, respectively). However, there were higher initial LA S in the 4C-view (p=0,0008) (Figure 5) and 2C-view (p=0,0011) (Figure 6). Initial SR values were higher in group 1 compared with group 2 in the 4C-view (Figure 7) and 2C-view (Figure 8): 4C-SR, 2,36±0,37 s-1 and 1,39±0,50 s-1 (p=0,0013); 2C-SR, 2,09±0,39 s-1 and 1,4±0,53 s-1 (p=0,0053).

Figure 3. Comparison of baseline LAV in patients without recurrent AF and with recurrent AF.
Abbreviations: RFA — radiofrequency ablation, AF — atrial fibrillation, LAV — left atrial volume.

Figure 4. Comparison of baseline LAVI in patients with and without recurrent AF.
Abbreviations: LAVI — left atrial volume index, RFA — radiofrequency ablation, AF — atrial fibrillation.

Figure 5. Comparison of initial longitudinal S in the 4C-view in patients with and without recurrent AF.
Abbreviation: AF — atrial fibrillation.

Figure 6. Comparison of initial longitudinal S in the 2C-view in patients with and without recurrent AF.
Abbreviation: AF — atrial fibrillation.

Figure 7. Comparison of initial longitudinal SR in 4C-view in patients with and without recurrent AF.
Abbreviation: AF — atrial fibrillation.

Figure 8. Comparison of initial longitudinal SR in 2C-view in patients with and without recurrent AF.
Abbreviation: AF — atrial fibrillation.
The contribution of initial LA S and SR values on the prognosis of developing LA reservoir dysfunction and, as a result, AF recurrence after catheter RFA was assessed.
According to the ROC analysis (Figure 9-11), the optimal cut-off values for initial longitudinal LA S in the 4C-view was S of 20,7% (AUC, 0,976±0,030; p=0,001), in the 2C-view — S of 19,2% (AUC, 0,964±0,041; p=0,001). The optimal cut-off values for initial LA SR in the 4C-view was SR of 1,8 s-1 (AUC, 0,958±0,047; p=0,001), in the 2C-view — SR of 1,75 s-1 (AUC, 0,899±0,081; p=0,005) (Figure 12, 13).
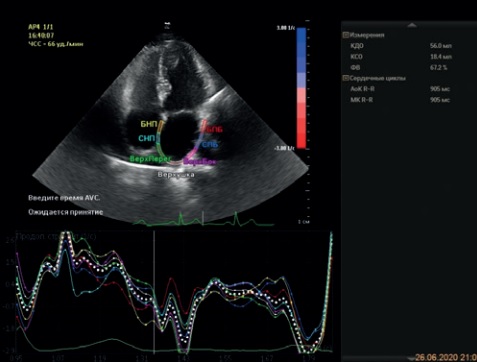
We present clinical examples showing the obtained graphs of longitudinal S (Figure 14 A) and SR (Figure 14 B) in a patient with effective RFA. In clinical practice, under time pressure, a physician is able to quickly and reliably assess the decrease in LA mechanics using the strain and strain rate curves in four-chamber and two-chamber views (Figure 15 A, B).

Figure 9. ROC-curve for predicting sinus rhythm maintenance after RFA depending on longitudinal LA S in 4C- and 2C-views.

Figure 10. AUC for predicting sinus rhythm maintenance after RFA, depending on longitudinal LA S in 4C- and 2C-views.

а. Наименьшее пороговое значение — минимальное наблюдаемое проверяемое значение минус 1, а наибольшее пороговое значение — максимальное наблюдаемое проверяемое значение плюс 1. Все остальные пороговые значения — средние двух последовательных упорядоченных наблюдаемых проверяемых значений.
Figure 11. Curve coordinates for predicting sinus rhythm maintenance after RFA, depending on longitudinal LA S in 4C- and 2C-views.

Figure 12. ROC-curve for predicting sinus rhythm maintenance after RFA, depending on LA SR in 4C- and 2C-views.

Figure 13. AUC for predicting sinus rhythm maintenance after RFA, depending on LA SR in 4C- and 2C-views.

Figure 14. Longitudinal LA strain in reservoir phase (A) and strain rate (B) in 4C-views in a patient with effective RFA.

Figure 15. Longitudinal LA strain in reservoir phase (A) and strain rate (B) in 4C-view in a patient with ineffective RFA.
Discussion
A lot of papers are devoted to the study of LA remodeling in major cardiovascular diseases complicated by AF development. At the same time, long-term arrhythmia leads to irreversible LA abnormalities in the form of fibrogenesis. As it is known, LA fibers can be highly stretched and lengthened in the reservoir phase. At the same time, LV contracted. Mitral annulus plane moves from cardiac base to the apex, which facilitates the suction of blood from the pulmonary veins in LA reservoir phase. Thus, 40% of blood volume filling the LV is provided. Then, when the mitral valve opens, blood passively flows into the LV in the early diastole phase by pressure gradient (35% of LV filling volume) — LA conduit phase. During the LA systole, the remaining blood volume (25% of LV filling volume) is actively squeezed into LV cavity — LA contractile phase [11][13].
Since the LA reservoir phase reflects the LA mechanics, it is likely that with an increase in LA fibrosis severity, the LA peak positive S and SR will worsen. In turn, the catheter RFA can lead to local fibrosis in the area of ablation scar, which, in turn, can reduce LA longitudinal S. Given the ambiguous dynamic changes in LA geometry and mechanics after RFA, it seems important to determine the predictors of AF recurrence.
When analyzing a few Russian and foreign works based on speckle tracking echocardiography, a significant scatter of threshold LA S values in different phases was revealed [11]. This circumstance is probably due to: 1) the use of different software (Soft) of ultrasound systems, which provides one of two possible approaches, registering the LA strain, either from the onset of the QRS complex (first positive peak — in LA reservoir phase), or from the beginning of P wave on the ECG (first negative peak corresponding to LA systole); 2) LA S values obtained during follow-up from 3 months to 1,5 years after RFA [2][9].
Agelaki M, et al. followed up 39 patients with paroxysmal AF (men, 74%) who underwent RFA and noted that 64% of patients maintained sinus rhythm that 1 year after the procedure [4]. The threshold value of initial LA S in the reservoir phase was 23,7% (AUC, 0,794). In addition, the authors noted that the baseline LA value in the contractile phase and the patient’s age (mean age, 63,5 years in patients with recurrent AF versus 54 years in patients without recurrent AF) became independent predictors of AF recurrence.
After the RFA itself, the dynamics of LA volume and S, as found by the Korean scientists, is ambiguous within 12-month follow up [2]. The study included 99 patients (mean age, 58,0±8,2 years; men, 75 patients (74,7%)), 59 of which had paroxysmal AF (60%) and 40 — persistent AF (40%). AF recurrence were observed in 5 out of 59 patients (8,5%) with paroxysmal AF and in 10 out of 40 patients (25%) with persistent AF.
LAVI, measured by 3D echocardiography and correlated with body surface area, increased after 1 day, decreased after 3 months, and then increased again after 1 year, but was lower than that before RFA. The dynamics of LA contractility during 3D echocardiography (LA ejection fraction) was similar to volume changes, but these parameters changed more pronounced in patients with persistent AF than in patients with paroxysmal AF.
Initial LAVI measured on 3D echocardiography was an independent predictor of AF recurrence after RFA, and the cut-off value was 44,13 ml/m2. Based on the findings, Hwang J, et al. concluded that even 3 months after RFA, the LA structural remodeling continued, and the changes were more pronounced in patients with persistent AF [2].
Mirza M, et al. found that among 63 patients aged 63±10 years with paroxysmal (75%) and persistent (25%) AF, 34/63 patients (54%) retained sinus rhythm after 18±12 months [8]. Initially, there were no differences between patients with recurrent AF and patients with effective RFA in clinical characteristics of patients, LA and LV volumetric parameters, Doppler evaluation of diastolic function, and filling pressure.
However, baseline LA ejection fraction, global and local systolic and diastolic S, SR were decreased in patients with recurrent AF. The authors noted that there was a decrease in the initial peak longitudinal S of LA lateral wall in the reservoir phase in patients with ineffective RFA compared with patients with sinus rhythm (11±7% vs 20±4%; p=0,007) and SR (0,9±0,4 s-1 vs 1,3±0,6 s-1; p=0,01) [8]. Thus, the baseline S of LA lateral wall is proposed as a predictor of AF recurrence after RFA, regardless of increase in the baseline LA size.
Schneider C, et al. described 118 patients with AF (74 with paroxysmal AF, 44 with persistent AF) who were referred for RFA. Of 118 patients, 82 (69%) maintained a stable sinus rhythm for 3 months [9]. Atrial characteristics after catheter RFA significantly differed in patients with paroxysmal AF (SR, 2,5 s-1, LA S in reservoir phase, 30%) from patients with persistent AF (SR, 2,3 s-1, LA S in reservoir phase, 25%). The best individual predictors of maintaining sinus rhythm were threshold SR values >2,5 s-1 for septum and inferior wall of LA, as well S in the reservoir phase >19,5% for inferior wall. S parameters were higher in patients with preserved sinus rhythm during 3-month follow-up, in contrast to patients with recurrent AF (p=0,001).
According to Hsu P-C, et al. a decrease in longitudinal S in the reservoir phase was associated with the risk of cardiovascular events with a threshold LA S <16,5% [14].
Our results are consistent with foreign studies. The success rate of RFA was 63%. As it was shown, the RFA result did not depend on the age of patients and LA volumetric parameters. Our study confirmed that the efficacy of catheter RFA was directly related to baseline LA S values as a marker of possible myocardial fibrosis.
The influence of developing LA fibrosis on LA S has been proven by Korean researchers [15]. When comparing the LA biopsy results obtained from patients during surgery with speckle tracking echocardiographic data, Her Ae-Y, et al. showed that impaired LA S directly depends on fibrosis severity [15].
Kuppahally SS, et al. compared contrast-enhanced cardiac DE-MRI (Multihance, Bracco Diagnostic Inc) and speckle-tracking echocardiography (VVI Software) in patients with AF. Contrast-enhanced DE-MRI revealed LA fibrosis in the form of local enhancement sites [3]. Fibrosis severity correlated with strain and strain rate, and the study demonstrated an inverse relationship between LA fibrosis area and pulmonary artery S and lateral LA wall SR [3].
This association was more pronounced in patients with persistent AF compared with paroxysmal AF. Patients with persistent AF had greater LA wall fibrosis compared with patients with paroxysmal AF (22±18% vs 14±9%, p=0,04) and lower LA inferior septal wall (38±15% vs 27±15%, p=0,01) and middle lateral wall (45±14% vs 35±18%, p=0,03) strain [3].
According to this study, the peak lateral wall longitudinal S is well visualized and can be used as a LA fibrosis marker [3]. There was no significant difference in SR between patients with persistent or paroxysmal AF [3].
Interestingly, LA and S fibrosis did not depend on AF etiology, patient’s age, presence of hypertension, LV filling pressure, or mitral regurgitation severity. Regardless of the underlying etiology or AF duration, atrial fibrosis severity was the main determinant of arrhythmia severity in this cohort. Thus, non-invasive LA fibrosis imaging can help predict the risk of AF progression, become a guide to developing a treatment strategy and predicting its results in patients with AF [3]. In addition, in the future, this will make it possible to reveal latent LA abnormalities at an early disease stage, before the development of serious or irreversible impairment of systolic and diastolic LA functions.
Potential study limitations. A small sample size due to careful selection of patients according to inclusion and exclusion criteria, as well as early exclusion from the study. The follow-up period lasted 1 year. There was no possibility of verifying the probable LA fibrosis using cardiac magnetic resonance imaging or endomyocardial biopsy (it was not the aim due to the high MRI cost and invasiveness of endomyocardial biopsy).
Conclusion
A stable sinus rhythm 12 months after the RFA was maintained in patients with higher baseline LA S and SR levels. The baseline LA S and SR values have a high predictive value for AF recurrence in patients after RFA. In patients with effective RFA, LAV and LAVI decreased without changing the S and SR. There was no effect of LA reverse remodeling and improvement in LA S values in patients with recurrent AF after RFA.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Musin T.I., Bagmanova Z.A., Gareev D.A., Rudenko V.G., Zagidullin N.S. Prediction of sinus rhythm maintenance after radiofrequency ablation in patients with atrial fibrillation using speckle tracking echocardiography and dynamics of left atrial structural and functional parameters. Russian Journal of Cardiology. 2021;26(2S):4256. https://doi.org/10.15829/1560-4071-2021-4256
Copy




