Increased natriuretic peptides not associated with heart failure
Blntroduction. Biochemistry and physiology of natriuretic peptides
Laboratory diagnostics of cardiovascular diseases (CVD) is an integral component of a treatment and prevention strategy aimed at early diagnosis, improving the duration and quality of li fe of patients. The search for new laboratory CVD biomarkers and clarification of diagnostic potential of old ones still remain one of the priority research areas [1]. The current key CVD biomarkers used in routine clinical practice include cardiac troponin isoforms and natriuretic peptides (NP). For the first time, NPs and their properties became known in 1981 with the studies by de Bold AJ, et al. The peptide discovered by them in the atrial myocardium had endocrine properties — a natriuretic effect, as a result of which it was named atrial NP (ANP) or A-type NP. Another historical name for this peptide was auriculin because it was found in the atrial appendage. These studies initiated a close study of the hormonal role of the heart. Subsequently, in 1989, in the ventricular cardiomyocytes, brain NP (BNP) or B-type NP was found. It got its name due to the fact that its structure corresponded to the peptide found earlier in the pig brain. And, finally, the third C-type NP (CNP) was identified in 1991 also in pig brain [2]. Thanks to molecular genetic studies, it became clear that NPs are a family of genetically different, but structurally related peptides. They have a similar structural conformation. ANP and BNP are predominantly expressed and secreted by cardiomyocytes (mainly atrial), and therefore are of interest as biomarkers in patients with CVD. At the same time, C-type NP is mainly produced in the central nervous system, endothelium, bone tissue and reproductive system, and its value in cardiology has not yet been established [2, 3]. According to modern concepts, NPs have hormonal/endocrine (vasodilation, natriuresis, suppression of aldosterone and endothelin) and auto- crine/paracrine (antihypertrophic, antifibrotic and proangiogenic) effects [3].
NPs are synthesized as preprohormones on the ribosomes of the endoplasmic reticulum, after which they undergo a number of post-translational changes and are converted into mature peptide molecules (hormones). The main clinically significant features of NPs are presented in Table 1.
Table 1
Characteristics of various NPs

Abbreviations: BNP — brain natriuretic peptide, ANP — atrial natriuretic peptide, NT-proBNP — N-terminal pro-brain natriuretic peptide.
The primary stimulus for ANP release from cardiomyocytes is atrial wall stretching [4]. Its blood plasma level in healthy people is approximately 20 pg/ml, while in patients with heart failure (HF) it is 10-100 times higher [5, 6]. The elimination halflife is very short (approximately 2 minutes) and its clearance mainly occurs in the lungs, liver and kidneys, with extraction ratios of 24%, 30% and 35%, respectively [7, 8].
BNP, unlike ANP, is stored in a minimum amount in secretory granules of ventricular cardiomyocytes and is secreted immediately in large portions after stimulation. Its plasma level in healthy people is approximately 3,5 pg/ml and can increase more than 100-fold in patients with HF [8, 9]. BNP has a precursor, the pro-BNP, which is cleaved into active BNP and inactive N-terminal pro-brain natriuretic peptide (NT-proBNP). The half-life of BNP is approximately 20 minutes, and NT-proBNP is approximately 120 minutes, which increases its diagnostic value [2]. The clearance of BNP depends mainly on neutral endopeptidase, while the NT-proBNP clearance depends on renal filtration [3, 5].
It has been shown that in patients with HF, the BNP level correlates with the severity of HF [2, 3, 9]. Using NP to confirm the HF and determine the prognosis or severity of the disease is regulated by the current international guidelines [8, 9].
One of the interesting directions of modern research is the study of cardiomarkers’ levels in other biological fluids, primarily in urine and oral fluid [1]. In this regard, the study of NP in the oral fluid may be of particular interest for the non-invasive diagnosis of HF. It was shown that the mean level of BNP in the oral fluid of patients with HF is significantly higher than that of healthy patients. Determination of salivary BNP can be a useful method for the diagnosis and follow-up of patients with HF [10].
Some factors affecting the NP levels
Large-scale multinational Breathing Not Properly study revealed that sex and age factors can have a significant influence on the NP levels [11]. In addition to biological factors, it depends on concomitant diseases such as obesity, coronary artery disease (CAD), acute coronary syndrome, atrial fibrillation (AF), renal failure, physical activity, cardiotoxic effects in the cancer therapy, taking sacubitril/valsartan, as well as false positive interference factors. Moreover, some conditions can either increase or vice versa decrease the NP concentration, which can lead to over- or underdiagnosis of HF. Below we will sequentially consider their impact on the level of NP and also discuss the main mechanisms underlying its increase.
It has been shown that body mass index (BMI) influences the choice of threshold values in the diagnosis of acute HF. Thus, Daniels L, et al. found that in the presence of obesity for the HF diagnosis, it is desirable to reduce the reference values of NP, and with a thin physique, on the contrary, to increase. In this study, the relationship between NP and BMI was revealed. Thus, the mean BNP levels in thin, overweight and obese patients with acute HF were 643, 462, and 247 pg/ml, respectively. And in patients without HF of a similar physique, the mean values were 52, 35, and 25 pg/ml, respectively. In general, to maintain the sensitivity of BNP in patients with severe obesity, a lower reference value should be used (?54 pg/ml), and for thin patients, a higher one (?170 pg/ ml), which increases the specificity for the diagnosis of HF [12]. McCord j, et al., sudying the effect of BMI on NP levels, confirmed the dependence of the BNP level on the patient’s weight. At the same time, when adjusting data on sex, age, severity of HF and kidney injury, BMI accounting did not bring additional diagnostic value [13].
A number of studies report that serum NP concentration in women is significantly higher than in men, however, the specific mechanisms underlying these features are still unknown [14-17]. In women, NP levels may be influenced by menstrual status, menopausal status, or use of hormone therapy [18, 19]. The most likely causes of sex differences in NP levels are thought to be the effects of sex hormones. It was reported that estrogens have a stimulating effect on NP formation, while androgens, on the contrary, inhibit it [14, 17, 20].
In the large study by Lam C, et al. with 4,056 patients, the serum concentration of NT-proBNP in men was significantly lower than in women in similar age groups, regardless of menopausal status and hormone therapy use. In addition, in women who received hormonal contraceptives, NT-proBNP was higher than in women who did not take them [16]. However, women taking hormonal contraceptives had the lowest levels of free testosterone and the highest concentrations of sex steroid binding globulin (SSBG). In both men and women, NT-proBNP increase was associated with a decrease in free testosterone and an increase in SSBG. These data are consistent with the hypothesis that androgens suppress NT-proBNP and suggest that differences in free testosterone may largely explain sex and hormonal differences in circulating NP levels [14]. In other words, there is a direct relationship between the NP levels and SSBG, and there is an inverse relationship between the NP levels and testosterone.
NPs in acute and chronic types of coronary artery disease
Myocardial ischemia leads to left ventricular (LV) systolic and diastolic dysfunction, which leads to an increase in NP concentration. In addition, when cardiomyocytes die, NPs will also be released into the blood. Thus, it should be expected that the greater the ischemia degree, the greater the NP increase. It is logical to expect that NP will be a valuable prognostic biomarker in both the short and long term in patients with acute coronary syndrome.
Patients with ST-segment elevation myocardial infarction (STEMI) who have serum BNP levels >80 pg/ml on admission have a 7,2-fold increase in 30-day mortality [21]. The ASSENT-2 and ASSENT-PLUS studies reported that the level of NT-proBNP at admission was an independent marker of one-year mortality in STEMI patients receiving thrombolytic therapy [22]. The same was observed in STEMI patients who underwent primary percutaneous coronary intervention [23-25]. In patients with non-STEMI m, the NL levels also have a high predictive value in predicting in-hospital and six-month mortality [26-28].
In chronic coronary artery disease, even the development of insignificant myocardial ischemia led to blood NP increase, while the predictive value of NT-proBNP was superior to that of BNP in predicting the risk of adverse events [29].
NPs and AF
It is well known that AF can cause dyspnea in the absence of HF and also lead to an increase in serum NPs. The PRIDE (ProBNP Investigation of Dyspnea in the Emergency Department) study included 599 patients who admitted to the emergency department with complaints of shortness of breath. To confirm/rule out the AF, all patients underwent electrocardiography. NT-proBNP levels were determined. AF was detected in 13% of patients at the time of admission, while their mean NT-proBNP level was significantly higher than in patients without AF (2934 pg/ml vs 294 pg/ml, p<0,0001). In patients without acute HF and with AF, the mean NT-proBNP level was significantly higher than in patients without AF (932 vs 121 pg/ml, p=0,02) [30].
In AF patients, it is recommended to use higher cutoff levels of BNP and NT-proBNP for the diagnosis of HF. The BACH (Biomarkers in ACute Heart Failure) study, which included 1445 patients with acute dyspnea, showed that the diagnostic value of BNP and NT-proBNP in acute HF decreased in the presence of AF [31]. The BNP threshold of 100 pg/ml had a specificity of 40% and 79% for the diagnosis of acute HF, respectively, in patients with and without AF. In patients with AF, a threshold of 200 pg/ml led to a significant improvement in the specificity of HF diagnosis compared to the conventional level of 100 pg/ml, with little sensitivity loss [31].
An inverse relationship was also noted between the NP levels and AF, namely, higher concentrations of NP are a risk factor for AF [32]. This may be due to a decrease in the effective refractory period in atrial cardiomyocytes [33].
The onset of AF in the early postoperative period is a poor prognostic factor. According to a metaanalysis with 5 studies, the level of NP can predict the risk of postoperative AF [32, 33].
NPs and renal failure
NPs are low-molecular-weight peptides, which they can pass through the glomerular filter and blood-saliva barrier into the urine and oral fluid, respectively [1, 10]. Ng L, et al. found that in patients with LV systolic dysfunction, urinary NT-proBNP levels were higher than in controls [34]. Glomerular filtration disorders, characteristic of chronic renal failure (CRF), are accompanied by an increase in serum NPs. However, Franz M, et al. found that in patients with impaired renal function, the NP excretion is increased. The progression of renal failure leads to a significant increase in the circulating NPs [35].
It has been shown that the BNP threshold for HF diagnosis can change if the glomerular filtration rate (GFR) is <60 ml/min/1,73 m2. The BNP level in patients with GFR <60 ml/min/1,73 m2 was 2-4 times higher than in patients with GFR ?60 ml/min/1,73 m2 [36, 37]. These findings are consistent with the the multinational Breathing Not Properly study of 1586 patients with acute dyspnea and a GFR <60 ml/ min/1,73 m2. It turned out that the optimal threshold values for BNP varied from 70,7 to 225,0 pg/ml for GFR ?90 and <30 ml/min/1,73 m2, respectively [38]. Thus, the NP level significantly increases with a GFR decrease, which has a significant impact on the diagnostic value in HF. It is likely that in patients with combination of HF and CRF, higher reference values should be used compared to patients without CRF. The most likely mechanism responsible for the increase in serum levels of NP in CRF is impaired NP elimination with urine.
NPs and exercise
Prolonged and intense physical activity can adversely affect the state of cardiomyocytes, as evidenced by a significant increase in cardiomarkers such as heart-type fatty acid-binding protein, copeptin, troponin I or T, NPs, and some others [39-42]. Schar- hag J, et al. found a NT-proBNP increase in 81 of 105 athletes after prolonged endurance exercise. The degree of NT-proBNP increase depended on the duration and severity of physical activity. The greatest increase in the NT-proBNP was observed in runners who 100-km running (mean value, 200 ng/l; 25/75 percentile, 115/770 ng/l). NT-proBNP was not associated with exercise-induced increases in troponin, but it was positively correlated with exercise time [39]. Considering that after a marathon run, the normalization of NT-proBNP occurs within 72 hours, it is assumed that this is based on a transient cardiomyocyte metabolism impairment [40].
Another hypothetical mechanism is the formation and release of membrane vesicles, in which the cytoplasmic proteins can also escape from the cardiomyocyte. A similar mechanism was first described by Schwartz P, conducting experiments on in vitro isolated cultured cardiomyocytes [43].
Medications affecting the NP levels
Myocardial injury by cardiotoxic drugs, in particular, chemotherapeutic agents used in the treatment of cancer, is accompanied by serum NP increase. NP concentration closely correlates with LV ejection fraction and LV global longitudinal strain after chemotherapy [44].
One of the factors contributing to a change in cardiomarker concentration is its metabolic characteristics, in particular, the half-life. Elimination of many cardiomarkers, including NPs, is carried out by blood filtration in the kidneys. The degradation of protein molecules is mediated by enzymes inside and outside the cell. To date, it is known that NPs circulating in the blood are cleaved by neprilysin, which belongs to the class of zinc-dependent metalloproteases. When ne- prilysin is blocked, the BNP molecules are not cleaved and circulate in the blood (accumulate) longer, and their concentration increases [45, 46]. Sacubitril in combination with the angiotensin receptor antagonist valsartan inhibits neprilysin, thereby increasing the blood concentration of BNP. In addition to the diagnostic value, this also has a therapeutic effect, since NPs have a beneficial effect on the myocardium of HF patients [45]. At the same time, the neprilysin does not degrade NT-proBNP, therefore, inhibition of neprilisin with sacubitril/valsartan does not affect the NT-proBNP concentration in any way. Therefore, in patients taking sacubtril/valsartan, it is recommended to determine NT-proBNP for diagnostics [45, 46]. Reasons for a false positive increase in NPs A false positive increase in NPs may be due to the heterophile antibodies [47-49]. The interference mechanism is associated with the effect of heterophile antibodies on the immune reaction between the diagnostic anti-NP antibody and the corresponding antigen. However, the number of cases described in the literature on false positive NPs due to heterophile antibodies is significantly lower than the number of cases described in terms of the effect of heterophile antibodies on cardiac troponins. Solter P, et al. revealed the effect of canine heterophile antibodies on enzyme immunoassay in the BNP determination [47]. Collin-Chavagnac D, et al. described a clinical case of a false-positive increase in NPs caused by monoclonal antibodies of the IgM class in a 75-year-old patient with Waldenstrom macroglobulinemia [48]. In this disease, tumor proliferation of B-lymphocytes and hypersecretion of monoclonal IgM are noted, which cause interference in immunochemical laboratory tests. Rheumatoid factor can be another reason for a false-positive increase in NPs [49]. Additional factors affecting the results of laboratory determination of NPs may be preanalytical errors leading to hemolysis, lipemia and blood clots [1, 50, 51]. Such elements adversely affect the optical density of the analyzed solution, which is then used to calculate the concentration of NPs.
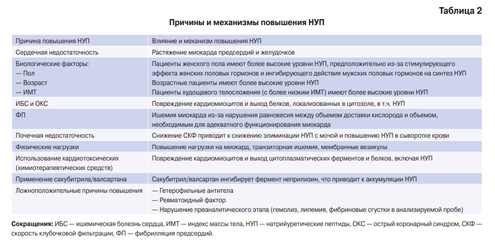
The main reasons and mechanisms for increasing the NPs considered above are presented in Table 2.
Table 2
Causes and mechanisms for NP increase

Abbreviations: CAD — coronary heart disease, BMI — body mass index, NPs — natriuretic peptides, ACS — acute coronary syndrome, GFR — glomerular filtration rate, AF — atrial fibrillation.
Conclusion
NPs are valuable biomarkers widely used for the diagnosis and prognosis of a number of CVDs. An increased NPs in HF occurs due to cardiac wall stretching, which stimulates the NP secretion by cardio- myocytes. In addition to HF, there are a number of reasons for the increase in NPs, which may be based on many other mechanisms not associated with myocardial stretching. According to some reports, in addition to blood serum, NPs are present in the oral fluid and urine. Further study is of both practical and research interest.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Chaulin A.M., Duplyakov D.V. Increased natriuretic peptides not associated with heart failure. Russian Journal of Cardiology. 2020;25:4140. https://doi.org/10.15829/1560-4071-2020-4140
Copy