Fundamental and practical aspects of coronary artery calcification
In recent years, the problem of coronary artery calcification (CAC) has been of concern to many specialists. Interventional and cardiovascular surgeons consider this problem from the perspective of the specific coronary anatomy, which presents difficulties in selecting the revascularization method, and often specifies the failure of percutaneous coronary intervention (PCI) [1]. Cardiovascular surgeons consider CAC as a factor of a possible aorta calcification associated with the difficulties when performing direct myocardial revascularization [2]. Radiologists focus on the need to choose the most informative and accessible method for detecting and quantifying CAC [3]. Cardiologists consider patients with CAC as a group of very high cardiovascular risk not only due to coronary artery lesion, but also due to additional comorbidity in these patients — bone, renal, metabolic [4][5], and consider the need for medication intervention in this process [6]. However, the largest number of publications on CAC in recent years has been devoted to the discussion of developmental pathways, the choice of the most sensitive and specific biological markers, as well as approaches to managing the risks of its development [6][7]. It should be noted that the lack of a unified point of view on these important issues, on the one hand, leads to dissatisfaction in the available research data, contradicting each other, on the other hand, it is an incentive to conduct new studies.
This review is devoted to modern papers on CAC, relevant points of view on the pathways, and clinical significance of CAC based on our own studies carried out at the Research Institute for Complex Issues of Cardiovascular Diseases.
Atherosclerosis and arterial calcification are interrelated synchronous pathological processes. It is believed that atherogenesis at all stages is in one way or another accompanied by impaired calcium and phosphorus metabolism and calcium deposition in the atherosclerotic plaque or in the arterial media [8]. At the same time, there is still no consensus about whether arterial calcification is the end stage of atherosclerosis, or is the development of CAC possible at its initial stages? Modern imaging technologies demonstrate that calcium plays a different role throughout the life of an atherosclerotic plaque. CAC can be a reflection of various pathological conditions, being present during the formation of an unstable, vulnerable plaque and during periods of its stabilization — delipidation, for example, with statin therapy. Therefore, at different steps of atherogen- esis, the clinical consequences of CAC can be very variable and diverse [3].
At present, there is no consensus on the extent to which calcification of an atherosclerotic plaque can provoke or prevent its rupture. For many years, the generally accepted opinion was that a calcified plaque is a factor of atherosclerosis stability [9]. However, the notion that CAC prevents plaque destabilization is currently being revised. It has been shown on mouse models that large calcium deposits in the coronary arterial intima affected by atherosclerosis can be related with plaque rupture followed by myocardial infarction (MI) [10]. Another evidence of the unfavorable role of CAC on the plaque is the fact that patients with severe calcification have a higher platelet reactivity and, in general, a higher throm- bogenicity [11], which indicates the vulnerability of seemingly stable atherosclerotic plaques. In addition, using intravascular ultrasound (IVUS), it was shown that even microcalcification of the atherosclerotic plaque capsule increases its tension and provokes the instability. At the same time, not all calcifications in the arteries is associated with an increased cardiovascular risk; it is also necessary to assess the distribution and morphology of calcium in atheroma [12].
For a long time, the assessment of CAC using the calcium index (CI) when performing multislice computed tomography (MSCT) was used exclusively for stratification of cardiovascular risk in the general population. It was believed that in patients with documented coronary atherosclerosis, to assess the severity of disease, it is sufficient to determine only the anatomical features of coronary atherosclerosis, the presence and severity of stenosis. The clinical and prognostic value of CAC in patients with documented coronary artery disease (CAD) was not considered [13]. At the same time, in recent years, more and more data have been accumulating that CAC is diagnostically important additional information for patients with CAD. CAC is a reflection of the anatomical severity of coronary atherosclerosis, a factor of severe comorbidity status, and, possibly, an additional marker of the clinical severity and unfavorable outcome of the disease [6]. CAC detection is especially important when selecting the optimal method of myocardial revascularization.
CAC — the view of a cardiologist and therapist. Calcification of any arteries without severe hormonal disorders and end-stage renal failure is an objective marker of aging. An increase in the area of calcium deposits in the aorta, aortic valve cusps, and coronary arteries is recorded in persons over 60 years of age. Aortic calcification leads to a change in its elasticity, the development of left ventricular hypertrophy followed by heart failure. With aortic calcification, the pulse wave velocity, systolic blood pressure, and pulse pressure increase. Aortic valve calcification leads to the development of degenerative aortic stenosis. In the coronary arteries, calcium deposits reduce vasodila- tory effects and affect the stability of atherosclerotic plaques in one direction or another [3].
CAC is more commonly diagnosed in older men. At the age of 70 and older, CAC is detected in more than 90% of men and 67% of women [7]. At the same time, the severity of arterial calcification is higher in men compared to women up to the sixth decade of life, and then CAC has no sex differences [14]. The menopause onset is associated with a 3-fold increase in the risk of detecting arterial calcification [15].
A number of studies revealed racial differences in the frequency and severity of CAC, which can specify the differences in clinical manifestations and outcomes of atherosclerosis. In the Multi-Ethnic Study of Atherosclerosis (MESA) [16], 6814 individuals without a history of cardiovascular disease of various races (whites, African Americans, His- panics, Chinese) aged 45 to 84 years were assessed. The prevalence of CAC (Agatston score >0) in these 4 ethnic groups in men was 70,4%, 52,1%, 56,5% and 59,2% (p<0,001), and among women — 44,6%, 36,5%, 34,9% and 41,9% (p<0,001), respectively. After adjusting for age, educational level, lipid profile, body mass index, smoking, diabetes, hypertension, statin use, sex and location of the research center, the relative risk (RR) of CAC, compared with the white race, was: for Africans — 0,78 (95% confidence interval (CI) 0,74-0,82); for Hispanics 0,85 — (95% CI 0,79-0,91) and for Chinese — 0,92 (95% CI 0,85-0,99). In addition, it was shown that the CAC severity in explanted hearts was higher in whites than in African Americans for every decade of life [17]. Several possible explanations for the higher prevalence of CAC in whites have been proposed. One of the possible reasons is the close relationship of calcification with a decrease in bone mineral density inherent in white people. It is known that African Americans have higher bone mineral density than whites, and, as a result, less pronounced calcification of the arteries. Another explanation is the specific racial differences in genes responsible for calcium and phosphorus metabolism [17]. However, the exact genetic mechanisms of these differences has not been identified.
Diabetes is considered as risk factor (RF) for CCA. Chronic kidney disease, which is a comorbidity of diabetes, is also such a factor [6]. The role of carbohydrate metabolism disorders in the development of CAC was evaluated in 2076 patients. A higher level of glycated hemoglobin was associated with any CAC progression (increase >10 Agatston units) during 5-year follow-up (RR=1,51 95% CI 1,16-1,96) and the advanced progression of CAC (increase >100 Agatston units) (RR=2,42; 95% CI 1,47-3,99) [18]. Previous studies of autopsy material from individuals with sudden death have shown that patients with diabetes, compared with those without it, have a higher percentage of calcified plaques and higher severity of calcification. At the same time, plaque macrophage and T-cell infiltration were also more intense, which indicates the presence of chronic intravascular inflammation in diabetes, provoking the progression of plaque calcification as a repair mechanism [19].
In our previous studies, in patients with multivessel coronary artery disease, only 10% had a minimal CAC calculated by the Agatston score; severe CAC was diagnosed in more than half of the patients examined. The relationship between the severity of coronary calcification and the severity of coronary atherosclerosis was determined, which indicates the pathophysiological parallelism of atherogenesis and CAC [20].
According to other studies, CAC in the general population is directly related to adverse outcomes and is a much more accurate marker of future events than other RFs. However, it is still not clear whether this is due to the calcified plaque itself as a source of future events, or whether calcified plaques are exclusively markers of global cardiovascular risk. The most promising is the data of coronary imaging in combination with the medical history and clinical characteristics of patients. Recent studies suppose that CAC cannot be considered as a qualitative variable (yes/no), but rather its quantity, type, location of calcification, volume and density matter [8]. Thus, the future of CAC identification and its risk interpretation lies with instrumental methods, which will simultaneously characterize both quantitative and qualitative characteristics of CAC.
CAC — the view of a diagnostic radiology specialist. Currently, MSCT is the main method for diagnosing and quantifying CAQ which has high sensitivity and specificity. The beginning of the practical use of high calcification density in coronary arteries was laid by Arthur Agatston, who presented in 1990 a protocol for quantitative assessment of CAС using electron beam tomography [21]. The standardized technique, which is based on the verification of structures with a density >130 Hounsfield units, is actively used at the present time, but already on modern high-resolution multislice tomographs [22].
Quantification of CAC is one of the key tools in assessing the risk of fatal cardiovascular events for patients with suspected CAD and with an intermediate pretest probability of CAD. The severity of CAC, assessed by MSCT, can be used as a screening examination for moderate-risk people and as an additional criterion for risk stratification in asymptomatic patients regardless of traditional CAD RF (hypertension, diabetes, lipid metabolism disorders, etc.). CI is the mathematical derivative of the calcification area on each tomographic slice and the factor of its X-ray density. The total CI score in Agatston units (AU) is formed by summing the scores of each calcified lesion in all coronary arteries (Figure 1). Depending on the obtained values of the total coronary artery CI, 4 grades are distinguished: minimal (1-10 AU), mild (11-100 AU), moderate (101-400 AU) and severe (>400 AU) calcification. It has been proven that CI is closely related to the severity of coronary atherosclerosis [23] and the risk of acute coronary events [24]. Risk stratification of fatal coronary events is carried out by comparing the absolute values of a respondent’s individual CI and the 75th percentile of the population-based CI adjusted for age and sex [25]. The percentage of coronary artery involvement in the calcification process, the so-called calcium coverage score, also characterizes the severity of atherosclerotic lesions and is associated with diabetes and dyslipidemia [26].

Figure 1. Cardiac MSCT with a quantitative assessment of coronary artery calcification. Voxels in the projection of coronary arteries with a density >130 Hounsfield units are defined as calcifications.
The limitations of quantitative assessment of CAC are due to, albeit low, but still potentially unfavorable radiation exposure of a patient. In addition, cardiac arrhythmia, the inability to lie motionless during the scan and hold the breath do not allow for a reliable assessment.
In patients with CAD, pronounced CAC is often detected during coronary angiography. Before the administration of a contrast agent, it manifests as radiopaque shadows [27]. Directly during angiography, calcium deposits in the arterial wall manifest as heterogeneous intraluminal abnormalities. In such a situation, it is necessary to differentiate CAC from the manifestations of intracoronary thrombosis, which is rather difficult to do in the context of conventional coronary angiography. Thus, the effectiveness of coronary angiography in assessing arterial calcium is not optimal, especially in patients with signs of stent restenosis. The study by Mintz GS, et al. [28] showed that coronary angiography detects calcium only in 38% of cases; the possibility of detection depends on the severity of CAC.
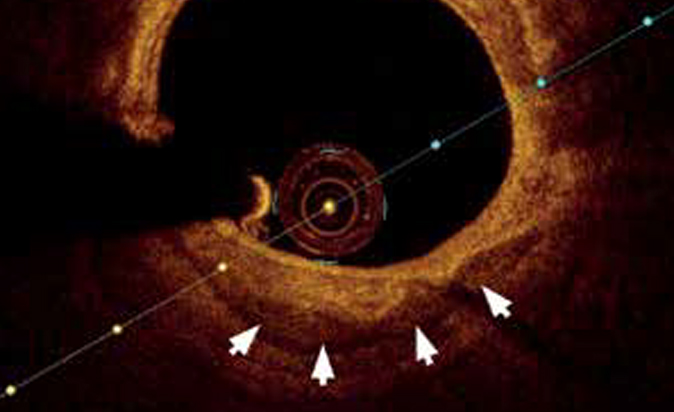
A more informative method for CAC detection is IVUS and optical coherence tomography (OCT), which makes it possible to comprehensively assess the calcium deposit. Since calcium causes a reflection of ultrasound, CAC usually appears in the IVUS image as a hyperechoic arc combined with a deeper acoustic shadow. An early IVUS autopsy study reported a sensitivity of 90% and specificity of 100% for detecting calcified atherosclerotic plaque or clustered microcalcifications and lower accuracy for detecting isolated microcalcifications (<50 ?m). These data were confirmed by further clinical studies that demonstrated a higher diagnostic value of IVUS compared with coronary angiography in CAC detection (73% and 38% of cases, respectively; p<0,001). However, the sensitivity of this method for the detection calcifications with a small surface area (<0,05 mm2) did not exceed 65% [29]. In addition, the limitation of IVUS in CAC detection is the ability to visualize only the anterior edge of the calcification without reliable information about the calcification thickness (Figure 2).

Figure 2. IVUS of the coronary artery. Eccentric calcium accumulations are represented by a hyperechoic signal from dense deposits (white arrows) combined with a deeper acoustic shadow (asterisks) that represents histopathological calcium.
A partial solution to the issue of quantifying the CAC is an integral indicator based on the calculation of calcification arc and length. But this approach does not reflect the true calcification given its depth. Thus, OCT has advantages in CAC detection in the form of a more accurate quantitative assessment of calcified plaque. In OCT, CAC is manifested as a low-signal area with a sharp luminal border [30]. OCT also provides data on the calcification area, thickness, length, and volume of calcification in three-dimensional space [31][32] (Figure 3).

Figure 3. On the OCT image of the coronary artery, calcified areas are represented by a low-signal area with a sharp luminal border (arrows).
It has been proven that these characteristics of CAC allow predicting the success of balloon angioplasty and coronary stenting. It should be noted that IVUS and OCT methods can also be used to assess the end result of stenting, and the information content of OCT is higher [33].
Thus, there are currently various methods for detecting CAC. At the stage of cardiovascular risk assessment, the method of choice is coronary artery MSCT, in patients with documented CAD — IVUS and OCT, which allow one to quantitatively characterize the length and thickness of calcification, the involvement of the distal areas. Nevertheless, the high availability and non-invasiveness of MSCT make this method promising for CAC assessment even in patients with CAD. Thus, in a cohort of patients with multifocal atherosclerotic lesions, ectopic coronary artery calcification according to MSCT is observed in 93% of patients, while most of them (73,5%) have moderate (101-400 AU) and severe (>400 AU) coronary calcification [4].
CAC — the view of an endovascular and cardiovascular surgeon. CA is one of four anatomical markers of the technical complexity of coronary interventions [1]. It is CAC that is associated with the maximum risk of intraoperative complications and long-term cardiovascular events.
Bourantas CV, et al. [34] showed that in PCI, patients with CAC are less likely to have complete myocardial revascularization than patients without CAC (48% vs 55,6%; p <0.001) and have a higher mortality risk during follow-up (10,8% vs 4,4%; p<0,001). It is important to note that the relationship between CAC and unfavorable outcome does not depend on the clinical manifestations of CAD and the type of implanted stents [27][35][36]. In the pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trials, PCI performed in patients with moderate to severe CAC were associated with a 62% increase in stent thrombosis and a 44% increase in ischemic target lesion revascularization [27].
Adverse clinical outcomes recorded in patients with CAC are associated with both comorbidities and increased technical complexity of PCI [37]. Typically, these lesions are less malleable with predilation. In patients with severe CAC, inadequate preparation of the calcified zone of the coronary artery for stent implantation increases the risk of stent loss, insufficient dilatation, or destruction. With pronounced CAC, the likelihood of no reflow phenomenon, dissection and perforation of the coronary artery during PCI is high. Upon implantation of drug-eluting stents (DES), the presence of CAC can damage the polymer on the stent and interfere with drug release [38].
Patients with severe CAC during PCI are characterized by higher values of markers of myocardial damage in the periprocedural period, which are a reliable criterion for an unfavorable long-term outcome of the disease [39]. It is known that the factors of a high probability of myocardial injury during PCI are advanced age and renal dysfunction, which reflects a patient with CAC [40]. It has been suggested that patients with higher severity of CAC should receive more intensive antithrombotic therapy after the procedure [41].
Coronary calcium is a marker of an unfavorable course of the postoperative period after coronary artery bypass grafting (CABG). One of the factors determining an unfavorable prognosis in this category of patients is severe calcification of the aorta, aortic valve, mitral annulus, which has an independent effect on the prognosis. There are very few studies on the effect of preoperative CAС on outcomes after CABG. In one of them, Ertelk K, et al. showed that CAС detected before CABG is an independent predictor of coronary events during 12 month follow-up [42]. During the first month after CABG, patients with severe CAC had perioperative MI 1,5 times more often than those without CAC. The authors of this study explain the presented results by the fact that CAC is associated with poor distal bed, endothelial dysfunction, and distal embolism. In addition, arterial calcification complicates the vascular anastomoses, increases the cardiopulmonary bypass duration, and reduces the likelihood of complete coronary revascularization. Coronary calcium, as well as aortic calcification during CABG, can increase the risk of bleeding and require more blood transfusion. CABG may reflect more severe atherosclerotic lesions in other vascular regions, which has an independent effect on CABG results. However, the low frequency of events over a short-term follow- up period in this analysis did not allow for firm conclusions.
In another study evaluating the role of CAC in the prognosis of patients undergoing CABG, one of the reasons for the unfavorable outcome after myocardial revascularization, the authors note the more frequent development of vein graft calcification in patients with baseline calcification of native coronary arteries [43].
Our study assessed the clinical and prognostic significance of CCA in patients with CAD after CABG. According to MSCT, more than half of the patients had signs of severe CAC [20]. The risk of adverse cardiovascular events (fatal and non-fatal acute vascular events and recurrent angina) within three years after CABG was associated with baseline high values of bone metabolism biomarkers (osteocalcin and parathyroid hormone) [44].
A retrospective analysis of the SYNTAX trial presents 5-year results of CABG in patients with varying grades of CAC assessed by coronary angiography. The authors concluded that severe CAC in patients with CABG, in contrast to patients with PCI, is not associated with a higher risk of incomplete revascularization and is not an independent predictor of mortality from MI and other cardiovascular events, but is an independent predictor of all-cause mortality. Higher mortality rates were observed in the period from 1 to 5 years after surgery, rather than during the first postoperative year. There were no differences in the incidence of acute coronary events, which meant that there was a higher baseline cardiovascular and renal comorbidity in patients with CAC These results considered that CABG is more effective method of myocardial revascularization for patients with CAD and severe CAC than PCI. However, the patient with CAC remains at high risk of unfavorable outcome after CABG [45].
CCA — the view of a clinical pathophysiologist. The importance of assessing calcification in terms of clinical presentation and prognosis specifies the relevance of studying biomarkers reflecting calcification. For many years, the calcification of an atherosclerotic plaque was considered as a passive, degenerative phenomenon with the mechanisms underlying the bone tissue formation [46]. At the same time, in recent years, a concept has been created that characterizes О^С as an active process, which is based on a systemic inflammatory response, typical for patients with metabolic syndrome or with renal dysfunction [3][47].
In general, CAC is the deposition of mineralized calcium in the endothelium or in the intercellular space of coronary artery media. It is assumed that lysosomes, mitochondria, intercellular substance (glycosaminoglycans), elastic and collagen fibers can be a matrix for calcification [48]. Ectopic vascular calcination usually consists of bone-like components: phosphates, calcium salts, hydroxyapatite, type I collagen, osteopontin, bone morphogenetic protein, osteocalcin, osteonectin, and matrix Gla protein [49]. This is facilitated by a number of factors in the initiation and progression of atherosclerosis: dyslipidemia, activation of oxidative stress markers (C-reactive protein), interleukins and growth factors. This, in turn, leads to endothelial dysfunction, a local increase in metalloproteinase levels, and the acivation of the RANKL/RANK/ OPG system and release of cathepsins with the formation collagen fibers as centers of future calcification in the atherosclerotic plaque. It is assumed that these are the common pathways of aortic and coronary calcification, as well as disorders of bone mineral density [50].
Factors of subclinical inflammation play a key role in the development of both CAC and bone mineral density disorders [51]. Inflammatory reactions are local (tissue) and systemic in nature; the general proinflammatory response is well reflected by an increase in C-reactive protein and a number of interleukins (-1, -6, -12, -18). The high activity of systemic inflammation with atherogenesis is persistent and leads to fibrosis and calcification in the intercellular subendothelial space of arteries [52]. In parallel, osteoclasts are activated in the bone tissue and complex hormonal changes are observed. In bone, the high rate of tissue inflammation is reflected in the activation of RANK/RANKL/ OPG system and inhibition of anti-inflammatory factors such as fetuin-? [53]. In the early stages of CAC, inflammatory cytokines activate osteogenic differentiation and mineralization of the vascular wall; at next stages, an increase in mineralization intensity is accompanied by a decrease in the content macrophage levels and further destruction of bone tissue [51]. The markers of bone destruction persistence include osteocalcin, calcitonin, cathep- sin, an increase in insulin level, a decrease in levels of androgens in men and estrogens in women [54]. The same markers were shown to be associated with the severity of CAC and aortic calcification [55]. According to our own data, the common pathogenetic factors for atherocalcinosis and osteoporosis in men with CAD were low levels of vitamin D and ionized calcium, increased levels of alkaline phosphatase, phosphorus and osteocalcin [5].
Therefore, it is relevant to discuss the role of lipid- lowering therapy in the development of CAD and osteopenia. According to a number of authors, the use of statins is associated with the stabilization of atherosclerotic plaque due to an increase in fibrous capsule density and the number and size of calcifications in the plaque [56]. At the same time, CAC necessarily passes the vulnerability stage, when the risk of rupture and erosion of the capsule is especially high due to calcification foci increase. The effects of statins on osteopenia are the subject of scientific debate. A number of authors discuss possible sex differences in the effects of statin therapy on osteoporosis. Nevertheless, a significant anti-inflammatory effect of statins has been proven, both in terms of local reactions and systemic ones [57]. Some authors suggest, in addition to statins, the use of bisphospho- nates and chelating agents to slow down the bone resorption and CAC progression [58].
Thus, the processes and mechanisms of CAC are a difficultly regulated pathophysiological phenomenon, which simultaneously reflects the activity of atherogenesis and disorders of bone mineral density. Identification of informative molecular markers and factors will make it possible in the future to develop effective strategies for medication managing the risk of its progression and individual prevention programs to improve the quality of life and life expectancy in patients with CAD.
Conclusion
The search for new mechanisms responsible for development and progression of atherosclerosis is urgent. CAC is probably one of the areas, the development of which can help in determining the most important diagnostic criteria for the severity and prognosis of CAD. Identification of the most sensitive biomarkers of CAC will be the basis not only for additional risk stratification of CAD and assessment of comorbidity, but also for the search for promising targets for prevention and treatment of atherosclerosis.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Barbarash O.L., Kashtalap V.V., Shibanova I.A., Kokov A.N. Fundamental and practical aspects of coronary artery calcification. Russian Journal of Cardiology. 2020;25(3S):4005. https://doi.org/10.15829/1560-4071-2020-4005
Copy