Renal dysfunction in patients with pulmonary embolism: data from the SIRENA register
Pulmonary embolism (PE) is one of the most common urgent cardiovascular diseases (CVD) [1]. Despite significant advances in its diagnosis and treatment, the incidence of adverse outcomes remains high [2][3].
Currently, in patients with acute CVDs, much attention is paid to cardiorenal relationships, which is due to the increased risk of development and/ or progression of renal dysfunction (RD) [4-8] and its adverse effect on prognosis [4][5][9]. These interactions have been described in patients with acute coronary syndrome (ACS) and heart failure and are described in the concepts of cardiorenal syndromes and cardiorenal continuum [10][11]. The prevalence of RD and its prognostic effect in PE is not well understood, and the available data are limited to a small number of studies, which are mostly retrospective [12]. Assessment of renal function in patients with PE, taking into account anticoagulant therapy, the dose and frequency of administration of which depend on creatinine clearance, is mandatory, but is often ignored by physicians [13]. The prevalence of RD in pulmonary embolism varies considerably, reaching 72% [14]. Taking into account that impaired renal function increases the risk of venous thromboembolism (VTE) [12][15], its prevalence is expected to be high.
One of the ways to reduce in-hospital mortality in PE patients is the early stratification of death risk. It is assumed that the assessment of renal function in pulmonary embolism, as with ACS, where the serum creatinine value is one of the factors for assessing unfavorable outcome (GRACE score), will improve the prognostic potential of current approach.
In Russia, until now, there have been no special multicenter epidemiological programs (registers) of PE, which would allow an objective assessment of RD prevalence in patients of the Russian population. Obtaining the data from the Russian multicenter PE register SIRENA makes it possible for such an analysis.
To assess the prevalence, severity and prognostic value of RD in Russian patients with PE, as well as to determine the RD significance as a marker that improves the predictive ability of current risk stratification systems.
Material and methods
This retrospective work is based on the Russian multicenter observational prospective register SIRENA, which was carried out in 20 hospitals in 15 cities of Russia (Birobidzhan, Kazan, Kemerovo, Maykop, Nizhny Novgorod, Perm, Ryazan, Samara, St. Petersburg, Sochi, Tver, Tomsk, Ulyanovsk, Ulan-Ude). Data on managing the register are described in detail in previous publications [16]. The register included all sequentially hospitalized adult patients with data suggestive of pulmonary embolism, as well as those who died in the hospital, in whom the diagnosis was established by autopsy. The recruitment of patients was carried out from April 2018 to April 2019. The study protocol was approved by local ethics committees. All alive subjects signed written informed consent. The following parameters were taken into account in each patient: demographics, symptoms at admission, risk factors (RF) of VTE, prehospital treatment, results of investigations, therapeutic management, complications, and hospitalization outcome.
In the previous papers, data on subjects with intravital diagnosis of PE were presented [16]. In the present work, patients with a postmortem diagnosis were added to the analysis. Since the article regards issues of RD, the study cohort included only those patients whose serum creatinine level was recorded. Renal function was assessed by the glomerular filtration rate (GFR) estimated using the CKDEPI equation [17]. Taking into account limitations of the SIRENA register, when only one creatinine value was recorded for the entire hospitalization, it was difficult to differentiate chronic kidney disease (CKD) from acute kidney injury (AKI). So, only the presence of RD, which was diagnosed with GFR <60 ml/min/1,73 m2, was considered.
Risk stratification of early (in-hospital or 30-day) death was carried out in accordance with the 2019 European Society of Cardiology guidelines [18]. A high death risk was determined in patients with persistent hypotension and/or obstructive shock on admission [18]. In the remaining patients, the PE severity index (PESI) and the simplified severity index (sPESI) were calculated based on clinical and demographic characteristics [18].
The duration of hospital period was assessed. The analysis of in-hospital mortality was carried out. Adverse events that developed during the hospitalization were taken into account.
Statistical processing of the material was carried out using the STATISTICA 6.0 (StatSoft, Inc (USA)) and MedCalc 11.6 (MedCalc Software bvba (Belgium)) programs. The type of distribution was assessed using Shapiro-Wilk test. In the case of normal distribution, the data are presented as M±SD, where M is the arithmetic mean, SD — standard deviation. Otherwise, the data are presented as Me (IQR), where Me is the median, and the IQR is the interquartile range: 25th percentile — 75th percentile. Qualitative variables are described by absolute (n) and relative (%) values. The significance of differences between two groups of normally distributed unrelated variables was determined by the Student’s t test. Otherwise, the Mann-Whitney U test was used. To compare the two groups on a qualitative basis, Pearson’s ?2 test was used. To compare three groups or more for one independent criterion, the Kruskal-Wallis rank analysis of variance was used. To predict the likelihood of event, Cox proportional-hazards regression analysis was performed. ROC analysis was used to assess the accuracy of diagnostic method. Differences were considered significant at p<0,05.
Results
In total, 660 patients were included in the SIRENA register. Further, 56 subjects were excluded from the analysis due to no data on creatinine levels. As a result, the studied cohort consisted of 604 patients, of which 293 (49%) were men, 311 (51%) — women. The mean age was 64±15 years.
RD was detected in 320 (53%) subjects, while its severe form (GFR <30 ml/min/1,73 m2) was noted in every tenth (Figure 1). Prior CKD was registered only in 61 (10%) patients.

Figure 1. Distribution of PE patients depending on GFR.
Abbreviations: GFR — glomerular filtration rate, PE — pulmonary embolism.
The initial demographic and clinical characteristics, depending on RD presence, are presented in Table 1. Data on physical examination, diagnostic investigations and PE severity indices are described in Table 2, while in-hospital mortality and adverse events during hospitalization — in Table 3.
Among patients with impaired renal function, males predominated. They were older, more often had a history of diseases, which are mostly VTE RFs (CVD, myocardial infarction, stroke/transient ischemic attack, hypertension, heart failure, atrial fibrillation, diabetes, CKD, chronic obstructive pulmonary disease, cancer) (Table 1).
Table 1
General characteristics, history, RFs and clinical signs in patients with PE, depending on RD presence

Abbreviations: HTN — hypertension, MI — myocardial infarction, RD — renal dysfunction, CVD — cardiovascular disease, DVT — deep vein thrombosis, TIA — transient ischemic attack, PE — pulmonary embolism, HF — heart failure, AF — atrial fibrillation, CKD — chronic kidney disease, COPD — chronic obstructive pulmonary disease.
In the group of patients with RD, symptoms, such as hemoptysis, cough, chest pain were less common, while objective signs (cyanosis, cervical veins enlargement) were observed more often (Table 1). In the case of impaired renal function, a lower level of diastolic blood pressure, oxygen saturation and a higher respiratory rate were detected (Table 2). According to complete blood count of RD patients, the level of hematocrit and platelets was lower, and while white blood cell count was higher (Table 2). Echocardiography in patients with impaired renal function revealed a lower left ventricular ejection fraction and a higher pulmonary artery systolic pressure (Table 2).
To confirm PE, computed tomographic angiography was performed in 501 (83%) patients. Pulmonary scintigraphy and pulmonary angiography were used in 5 subjects for both (0,8% each). In patients with RD, contrast-enhanced x-ray investigations were used less frequently, while postmortem diagnosis was carried out more often (Table 2).
Table 2
Results of physical examination, diagnostic investigations and PE severity indices, depending on RD presence

Abbreviations: BP — blood pressure, RD — renal dysfunction, RV — right ventricle, RA — right atrium, PE — pulmonary embolism, RR — respiratory rate, HR — heart rate, PESI — pulmonary embolism severity index, sPESI — simplified pulmonary embolism severity index.
Seventy-one (12%) of 604 PE patients had a high death risk, which was determined based on hypotension and/or shock on admission. For the rest of subjects, PESI and sPESI were calculated in order to assess the death risk. Their level in patients with RD was significantly higher than in those without it (Table 2). Patients of low risk, depending on sPESI value, were divided into moderate and low risk groups: the first one included 364 (61%) patients, while the second — 164 (27%). The higher the death risk in patients with PE, the more often RD was diagnosed (Figure 2).

Figure 2. RD incidence in patients with PE, depending on the death risk.
Abbreviations: RD — renal dysfunction, PE — pulmonary embolism.
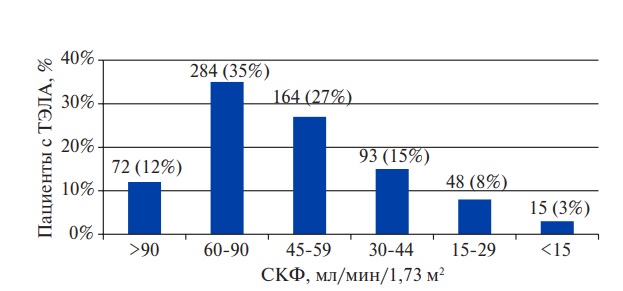
For the rest of the subjects, PESI and sPESI were calculated in order to assess the death risk. Their level in patients with RD was significantly higher than in those without it (Table 2). During the hospitalization, 107 (18%) patients died, while in the high-risk group — 32%, moderate — 20%, low — 7% (Kruskal-Wallis: H=23,47; p<0,001). The GFR level in deceased patients was lower than in the survivors (41,9 (27,1-58,3) and 61,1 (49,2-79,2) ml/min/1,73 m2, respectively; p<0,001), while the number of those who died increased stepwise as it declined (Figure 3). RD in deceased patients was diagnosed more often (Table 3), while the GFR ?50 ml/min/1,73 m2, identified by ROC analysis, reliably predicted hospital death with a sensitivity of 67% and a specificity of 72% (AUC=0,72; p<0,001).
In order to clarify the potential of assessing RD in death risk reclassification, the low risk subjects were divided into 4 groups: first group — patients with sPESI score of 0 and without renal dysfunction; second — patients with sPESI score of 0 and RD; third — patients with sPESI score ?1 and without impaired renal function; fourth — patients with sPESI score ?1 points and RD. In the case of sPESI 0 and sPESI ?1, impaired renal function led to at least a 2-fold increase in mortality, and its highest level was observed in the fourth group (Figure 4). Cox multivariate regression confirmed that RD is a predictor of in-hospital mortality (hazard ratio (HR), 3,41 (95% confidence interval (CI): 2,15- 5,41; p<0,001), regardless of presence of such known death risk reclassifiers as elevated troponin (HR, 1,31 (95% CI: 0,80-2,14; p=0,28)) and right ventricular dysfunction (HR, 1,23 (95% CI: 0,74- 2,04; p=0,42)) (Table 3).

Figure 3. In-hospital mortality in PE patients depending on GFR.
Abbreviations: GFR — glomerular filtration rate.

Figure 4. In-hospital mortality in PE patients depending on sPESI value and RD presence.
Notes: * — p<0,05 — significance of differences with non-RD group.
Abbreviations: RD — renal dysfunction, PESI — pulmonary embolism severity index, sPESI — simplified pulmonary embolism severity index.
During hospitalization, 222 (37%) patients with PE developed adverse events in the form of shock, bleeding, pneumonia, acute heart failure (AHF) and other rarer conditions. The incidence of these events in general did not depend on renal function, however, AHF was observed more often in subjects with PD (Table 3).
Table 3
In-hospital mortality and adverse events in patients with PE, depending on RD presence

Abbreviation: RD — renal dysfunction.
Table 4
Multivariate Cox regression for assessing in-hospital mortality RFs in patients with PE

Note: Chi-square model — 34-97, р<0,001.
Abbreviations: В – regression coefficient, SE — standard error, CI — confidence interval.
Discussion
In the presented study, we assessed RD in PE patients, which was considered as <GFR 60 ml/min/1,73 m2. This approach is often used when it is required to identify impaired renal function and study its effect on prognosis, but there is insufficient data to differentiate AKI from CKD [14][19][20]. Indeed, the lack of data on serum creatinine levels before the index event in urgently hospitalized patients is a recognized problem [12]. The use of estimated creatinine in this situation (corresponding to GFR of 75 ml/min/1,73 m2 by MDRD equation) as the initial one for diagnosing kidney injury is not considered a reliable method, since there is a possibility of bidirectional errors in identifying the incidence and severity of renal dysfunction: underestimation or overestimation of both AKI and CKD. In the context of analyzing RD types, attention should be paid to the fact that it is often impossible to establish which condition was earlier: acute cardiovascular disease or acute renal disfunction, since each of them can potentiate the development of the other [21][22].
Data on RD in PE are limited by several studies [12], while the incidence of this condition, according to largest literature review to date, ranges from 12 to 72% [14]. Such a large range of values is explained by the differences in characteristics of participants (age, race, sex, comorbidities, etc.) and the use of different equations for assessing GFR [14][20]. According to the Russian register presented by us, RD was detected in every second subject, which was comparable with prospective studies by Kostrubiec M, et al. (2012) [23] and Ouatu A, et al. (2015) [24], where GFR <60 ml/min/1,73 m2 occurred in 48% and 37% of cases, respectively. In patients with pulmonary embolism, renal function impairment may be associated with prior CKD, including not previously diagnosed, and AKI both de novo and in the form of AKI with prior CKD.
VTE is more common in CKD patients than in the general population [12][15]. The results of the analysis of 32 million discharge reports from hospitals covered by the Nationwide Inpatient Sample (NIS) database showed that PE risk increases as RD severity increases [15]. The incidence of pulmonary embolism increases with age [18], which, according to large registries, averages 68 years [3]. Patients with PE have a high incidence of concomitant diseases, which are themselves associated with impaired renal function [4]. In our study, hypertension was present in 2/3 of patients, diabetes mellitus — in 32%, CVD — in 27%, stroke/transient ischemic attack — in 12%, heart failure — in 25%, and atrial fibrillation — in 23%. Considering that signs of decreased GFR and/or kidney injury are detected in 13% of people in the population, and in every third person over the age of 60 [25], a high incidence of CKD in PE should be expected. According to our data, only 10% of patients had prior CKD, but this fact rather demonstrates an insufficient detection rate than an actual situation. According to some studies, mostly retrospective, the AKI incidence in patients with PE ranges from 5 to 30% [23][26][27].
According to the study, where the diagnosis was carried out according to the KDIGO criteria (2012), this complication was detected in 29,5% of patients [27]. There are following mechanisms leading to AKI in pulmonary embolism: renal macro- and microcirculatory hypoperfusion, renal venous hypertension and increased neurohumoral activation [18][23][28]. In addition, kidney injury can be caused by intravenous contrast administration during computed tomographic angiography, which is currently the main method for diagnosing pulmonary embolism. Some researchers attribute all AKI cases to contrast-induced nature with an incidence of up to 24% [29], but this approach contradicts the guidelines that require other causes of complications to be ruled out [5]. Recent data demonstrate that AKI risk after contrast administration is clearly lower than previously assumed, and does not exceed 2% with an initial GFR >30 ml/min/1,73 m2 [30].
RD is an important prognostic factor in patients with CVD and is associated with increased morbidity and mortality [4][31]. It has now been established that a decrease in renal function in PE is associated with an increase in 30-, 90-, 180-day and 1-, 2-year mortality, and these data were obtained not only in separate retrospective or prospective studies [24][32] but also in large multicenter studies [33] and meta-analyzes [34][35]. Papers on relationship between RD and in-hospital mortality in patients with pulmonary embolism are sporadic and contradictory. A retrospective analysis by Keller K, et al. (2017) demonstrated no association between creatinine levels and in-hospital death [36]. Similar data were obtained by Fabbian F, et al. (2013) in a study based on 24 thousand PE cases [37]. In a large metaanalysis, decreased renal function led to an increase in in-hospital/30-day mortality, but this association regards only AKI but not CKD [34]. In the SIRENA register, RD was diagnosed more often in the deceased patients, while GFR ?50 ml/min/1,73 m2 reliably predicted in-hospital mortality. Probably, such a result was due to the inclusion of patients who died in the hospital, in whom PE was diagnosed by autopsy, which increased the number of deaths.
In acute CVDs, RD is an important RF of unfavorable outcome and is included in the following prognostic scores: GRACE (ACS), MAGIC (AHF). The PESI used to stratify the risk of early death in patients with PE does not include parameters characterizing renal function. Currently, in order to overcome the limited accuracy of the system for assessing an unfavorable outcome in PE, reclassifiers have been developed that have proven their prognostic value: right ventricular dysfunction, markers of myocardial damage and hemodynamic stress [18]. In our study, RD was found to be a predictor of in-hospital death, regardless of elevated troponin levels and right ventricular dysfunction. GFR <60 ml/min/1,73 m2 was associated with a more than 2-fold increase in mortality, which was observed with both low (sPESI of 0) and moderate (sPESI ?1) PE severity, which demonstrates additional potential of RD in identifying patients with a high probability of an unfavorable outcome. It should be emphasized that, in contrast to complex approaches to mortality risk stratification, assessment of renal function using estimated GFR has significant advantages: it is affordable, easy to use and reproducible.
The mechanisms by which RD affects clinical outcome are rather multifactorial and include endothelial dysfunction, systemic inflammation, increased risk of bleeding/thromboembolic events, and limitations in the use of some treatment approaches [19]. Renal impairment is probably an integrated marker of high risk, reflecting the presence of not only previous CKD or newly developed AKI, but also comorbidities, since it tends to be combined with other cardiovascular RFs.
The limitation of this work is its retrospective design, due to the fact that the aim was formulated after the register end. In addition, the SIRENA design implied recording only one serum creatinine value in database without information on its dynamics and prehospital level. This did not allow assessing the basic renal function, differentiating CKD from AKI, and led to using term “RD”. The incidence of prior CKD reported by the researchers is probably underestimated due to emergency conditions of hospitalization and the lack of outpatient medical records. The study included 8% of patients with postmortem diagnosis of PE, which led to an increase in the relative number of deaths and could affect the results of assessing the prognostic significance of RD.
Conclusion
In patients with PE of the Russian population, there is a high incidence of RD, which is diagnosed in every second patient and is severe in 10% of cases. The presence of RD is associated with a significant increase in in-hospital mortality, while the risk of death increases with a decrease in GFR. The addition of RD, considered as a decrease in the estimated GFR <60 ml/min/1,73 m2, to the sPESI improves risk stratification and allows identification of patients at high risk of in-hospital death.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Menzorov M.V., Filimonova V.V., Erlikh A.D., Barbarash O.L., Berns S.A., Shmidt E.A., Duplyakov D.V. Renal dysfunction in patients with pulmonary embolism: data from the SIRENA register. Russian Journal of Cardiology. 2021;26(2S):4422. https://doi.org/10.15829/1560-4071-2021-4422
Copy