Effect of combined lipid-lowering therapy on atherosclerotic plaque vulnerability in patients with acute coronary syndrome (Combi-LLT ACS): randomized trial protocol
At the basis of coronary artery disease is, as a rule, atherosclerosis. A large lipid pool, thin fibrous cap, and pronounced inflammatory cell infiltration are important characteristics of atherosclerotic plaques, indicating a tendency to rupture and a high risk of fatal coronary events. Plaque destabilization is caused not only by lipid composition changes, but also by infiltration by immune inflammatory cells and degradation of vascular extracellular matrix, as well as an active inflammatory response and plaque neovascularization [1][2]. An important role in this process is the markers of matrix remodeling (matrix metalloproteinase-9 (MMP-9), tissue inhibitor of metalloproteinase 1 (TIMP-1)), myocardial fibrosis and vascular inflammation (galectin 3 (GAL-3), high-sensitivity C-reactive protein (hsCRP), neutrophil-lymphocyte ratio (NLR), neutrophil gelatinase-associated lipocalin (NGAL)) [3-14]. In recent years, the direction of cardiovascular imaging has been actively developing — intravascular ultrasound, high-resolution magnetic resonance imaging, positron emission tomography-computed tomography, optical coherence tomography (OCT), which allow one to define characteristics of vulnerable plaques in vivo [15].
Treatment of dyslipidemia as a major factor in the development of atherosclerosis is a key component of primary and secondary prevention of cardiovascular disease. To date, 3 following groups of drugs are actively used to reduce low-density lipoprotein cholesterol (LDL-C): 3-hydroxy-3-methyl-glutarylCoA reductase inhibitors (statins) as first-line drugs, an inhibitor of intestinal free cholesterol absorption (ezetimibe) is used as a second-line therapy in patients who either cannot tolerate statins or cannot achieve the target LDL-C level despite maximally tolerated statin therapy. If further reduction in LDL-C is required, a stepwise approach using the proprotein convertase subtilisin/kexin type 9 (and PCSK9) inhibitors alirocumab or evolocumab is re – commended, based on the results of the FOURIER and ODYSSEY OUTCOMES trials [16][17].
The aim of the study was to investigate the effect of high-dose combined lipid-lowering therapy (statins + ezetimibe vs statins + PCSK9 inhibitors) on plaque vulnerability characteristics, assessed using multislice computed tomography (MSCT) coronary angiography and OCT), as well as biomarkers in patients who after acute coronary syndrome (ACS).
Material and methods
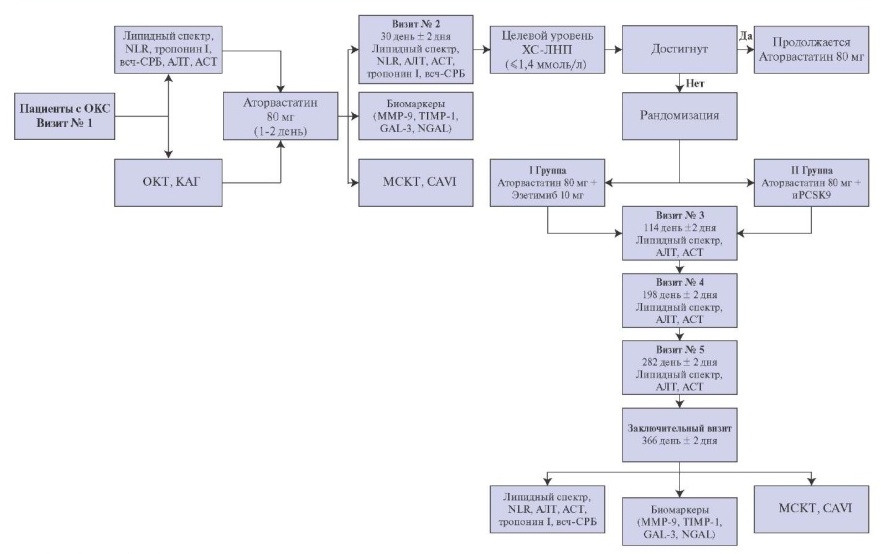
Study design. This open-label, prospective, randomized, single-center study is planned to include 120 patients admitted on an emergency basis with ACS (Figure 1). All patients will undergo percutaneous coronary intervention (PCI) of the infarctrelated artery (IRA), as well as intracoronary imaging with OCT of one or two non-IRAs. During hospitalization, patients will receive standard therapy for ACS according to guidelines, while statins will initially be prescribed at the maximum dosage (atorvastatin 80 mg/rosuvastatin 40 mg). Patients who showed high compliance and did not reach the target LDL-C values (>1,4 mmol/l) 1 month after the myocardial infarction (MI)/unstable angina, at visit 2 will be randomized into 2 groups. Group 1 patients will receive PCSK9 inhibitors (alirocumab 150 mg by subcutaneous injection once every 2 weeks or evolocumab 140 mg by subcutaneous injection once every 2 weeks) in addition to statin therapy at the maximum dosage. Group 2 patients will take ezetimibe at a dose of 10 mg in combination with the maximum dose of statins.

Figure 1. Study design.
Abbreviations: ALT — alanine aminotransferase, AST — aspartate aminotransferase, hs-CRP — high-sensitivity C-reactive protein, iPCSK9 — proprotein convertase subtilisin/kexin type 9 inhibitors, LDL-C — low-density lipoprotein cholesterol, CAVI — cardio-ankle vascular index, GAL-3 — galectin 3, MMP-9 — matrix metalloproteinase-9, NGAL — neutrophil gelatinase-associated lipocalin, NLR — neutrophil-to-lymphocyte ratio, TIMP-1 — tissue inhibitor of metalloproteinase 1.
The study complies with the Good Clinical Practice standards and the ethical aspects of the Helsinki Declaration of the World Medical Association, paragraph 15 of Art. 37 of the Federal Law № 323-FZ of 21 November 2011 on Basics of Health Protection of the Citizens in the Russian Federation. The study protocol was approved by the local ethics committee and is registered with ClinicalTrials.gov.
Inclusion criteria. Any sex; age of 18-75 years; acute coronary artery disease (unstable angina or MI) with at least one coronary artery stenosis requiring PCI; MI within last 24 hours; one or two nonIRA (coronary angiography <50% diameter and no need for revascularization within the next 6 months); not taking statins for at least 3 (6) months prior to ACS; not achieved target LDL-C level at the first visit; not achieved LDL-C level ?1,4 mmol/l at the second visit; signed informed consent.
Exclusion criteria: prior MI; prior revascularization (PCI/coronary bypass grafting); non-IRA stenosis ?50%; multivessel lesion, including significant left coronary artery stenosis, Simpson’s ejection fraction <40%; Killip III-IV; NYHA class heart failure III-IV; significant calcification or coronary artery tortuosity; limitations for OCT; severe renal and hepatic insufficiency; allergic reactions to iodine-based contrast agents; intolerance to statins, aspirin, P2Y12 blockers; patients who have previously received PCSK9 inhibitors and/or ezetimibe; treatment with systemic steroids or systemic cyclosporine within the last 3 months; collagenoses and inflammatory diseases; cancer within the last 5 years; elective surgery within 3 months; mental disorders; pregnancy; breastfeeding period.
Enrollment in the study. All patients admitted on an emergency basis with ACS will undergo blood and urine tests on the first day, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), troponin I, hs-CRP, lipid profile, coronary angiography followed by PCI-IRA, and intracoronary imaging with OCT of one or two non-IRA. Patients will receive standard therapy for MI/unstable angina during hospitalization and throughout follow-up according to clinical guidelines, with statins initially administered at a maximum dose of atorvastatin 80 mg/rosuvastatin 40 mg.
At the stage of selection for the study, the purpose and objectives are described to a patient who meets the inclusion/exclusion criteria; all the risks and benefits of participation are explained in detail. In case of consent to participate in the study, the patient signs 2 copies of the informed consent and receives one of them. The data of each participant in the study are entered into specially designed individual patient registration cards.
Randomization. Patients who showed high compliance and did not reach the target LDL-C values (?1,4 mmol/l) 1 month after the development of MI/unstable angina, at visit 2 will be randomized into two groups of 50-60 patients each. Group 1 patients will receive PCSK9 inhibitors (alirocumab 150 mg by subcutaneous injection once every 2 weeks or evolocumab 140 mg by subcutaneous injection once every 2 weeks) in addition to statin therapy at the maximum dosage (atorvastatin 80 mg/rosuvastatin 40 mg). Group 2 patients will take ezetimibe at a dose of 10 mg in combination with the maximum dose of statins. In addition, at visit 2, patients will undergo MSCT coronary angiography, assessment of the cardio-ankle vascular index (CAVI) and laboratory tests (blood for lipid profile, ALT, AST, troponin I, GAL-3, MMP-9, TIMP-1, hs-CRP, NGAL, NLR) [18][19].
Endpoints. Primary endpoint: reduction in plaque vulnerability in non-IRA. Secondary endpoints: death, stent thrombosis/restenosis, non-fatal MI, rehospitalizations with progressive angina, repeat revascularization; lipid profile (total cholesterol, LDL-C, HDL-C, triglycerides) changes against the background of maximum combination therapy with statin + PCSK9 inhibitors or statin + ezetimibe; dynamics of biomarkers of myocardial damage (Troponin I), inflammation (NLR, hs-CRP, NGAL, GAL-3) and matrix remodeling (MMP-9, TIMP-1).
Long-term follow-up. The total follow-up duration was 52 weeks; every month a telemedicine consultation will be provided, a visit to monitor the effectiveness and safety is planned every 3 months, where a blood test for lipid profile, ALT, AST, NLR will performed; in patients receiving PCSK9 inhibitors, intermediate monitoring will be performed every 2 weeks during the drug injection). At the final visit 12 months later, patients will undergo MSCT, assessment of the CAVI and laboratory status (lipid profile, troponin I, GAL-3, MMP-9, TIMP-1, hsCRP, NGAL, NLR).
The following adverse events will be analyzed when taking drugs: in the ezetimibe group — headache, abdominal pain, diarrhea, constipation, nausea, myalgia, increased ALT and/or AST >3 upper limits of normal (ULN), creatine phosphokinase (>5 ULN), thrombocytopenia; in the iPCSK9 group — injection site reactions (dryness, erythema, hematoma, hemorrhage, swelling, pain), allergic reactions — urticaria, neurocognitive disorders: impaired attention, memory impairment, disorientation, upper respiratory tract infections (nasopharyngitis), back pain, arthralgia, myalgia, headache, dizziness, diarrhea, abdominal pain, urinary tract infections, increased ALT and/or AST >3 ULN.
Statistical analysis. It is proposed to evaluate the comparable effect of the two therapy approaches in reducing the manifestations of plaque vulnerability in non-IRA. It is planned to use SPSS and Statistica packages to process the obtained results. The analysis will use parametric and non-parametric statistics. To assess the significance of independent predictors, it is planned to use a logistic regression model. Categorical variables will be specified as absolute numbers and percentages. Relative risks and 95% confidence intervals will be calculated using the two-way tabulation with a logarithmic approximation. Continuous variables will be presented as mean ± standard deviation or median and interquartile range. P<0,05 will be taken as the level of statistical significance.
Current status of the study. The study is currently recruiting patients. The study protocol is registered with ClinicalTrials.gov.
Discussion. Vulnerable plaques are a major cause of ACS and sudden death. Vulnerable plaques include any type of plaque with a high risk of thrombotic events and rapid progression, characterized by a thin fibrous cap, a large lipid pool, infiltration of inflammatory cells (especially macrophages), extracellular matrix remodeling, neovascularization, and plaque hemorrhage [1].
One of the likely mechanisms for preventing cardiovascular events may be the stabilization and/or regression of existing coronary plaques. The fibrous cap thickness can be assessed using OCT. The Lipid Rich Plaque and PROSPECT II studies confirmed the association of vulnerable atherosclerotic plaques with future ischemic cardiovascular events. In addition to various imaging modalities, biomarkers for inflammation (NLR, hs-CRP, GAL-3, NGAL) and matrix remodeling (MMP-9, TIMP-1) have been proposed to identify vulnerable plaques.
One of the most studied inflammatory molecules is C-reactive protein, which is produced by the liver in response to infection or tissue damage. The serum level of CRP <1,0 mg/l in the initial state increases 1 thousand times in the acute phase of the immune response. CRP can bind to oxidized or degraded complement-activating LDL and induce the expression of adhesion molecules, uptake of LDL-C by macrophages, and the production of chemokines that attract monocytes and other immunoinflammatory cells to the arterial wall. An elevated serum hs-CRP level reflects a tendency to plaque rupture and a high atherosclerotic burden [2][10].
NLR is an inflammatory marker, an increase in which is associated with an increased risk of cardiovascular events. Normally, the NLR value, as a rule, does not exceed 3,0. NLR is an important marker for assessing the prognosis of patients with cardiovascular disease, because it is little influenced by physiological conditions, and therefore provides an opportunity to assess inflammatory immune pathways (neutrophil count), as well as the body’s response to stress (lymphocyte count). One of the hypotheses for the relative mechanism of the increase in the NLR ratio is a decrease in lymphocyte count after programmed cell death (apoptosis) or the movement of lymphocytes from peripheral blood into the heart tissue with subsequent infiltration, which was found in patients with acute coronary artery disease. An increase in NLR is associated with the progression of atherosclerosis, since neutrophils and macrophages enhance phagocytosis and degradation of vascular tissue [8].
GAL-3 is a beta-galactosid-binding pleiotropic lectin released into the extracellular matrix by activated cardiac macrophages and regulates apoptosis, proliferation, inflammation, and fibrosis. A potential role for GAL-3 as a mediator of atherosclerosis has been proposed. Many studies have shown that GAL-3 promotes macrophage differentiation, foam cell formation, endothelial dysfunction and proliferation, and migration of vascular smooth muscle cells during atherogenesis [5][6].
MMP-9 is secreted by various cells, including cardiomyocytes, endothelial cells, neutrophils, macrophages, and fibroblasts, and correlates with plasma concentrations of interleukin-6, CRP, and fibrinogen, indicating that MMP-9 can predict adverse cardiovascular outcomes regardless of association with inflammatory markers. MMP-9 plays a role in the stability of atherosclerotic plaques by counteracting intima thickening. MMPs also lead to the destruction of the main extracellular matrix components, which causes plaque rupture [3][4].
MMPs are endogenously inhibited by TIMP, which are composed of four members (TIMP-1, -2, -3 and -4). TIMP-1 inhibits MMP-9 with high affinity. TIMPs are produced and secreted by fibroblasts, epithelial and endothelial cells and distributed among tissues. MMP activity is tightly regulated by endogenous TIMPs, and dysregulation of activity promotes extracellular matrix remodeling. An imbalance between MMP and TIMP levels leads to dysregulation of proteolytic activity and usually unfavorable extracellular matrix remodeling and is associated with the progression and instability of atherosclerotic plaques in coronary arteries along with unfavorable postinfarction fibrosis and subsequent heart failure.
However, to date, there are no reliable data on the possibility of using these biomarkers for the longterm monitoring of vulnerable plaques [3-14].
According to European Society of Cardiology guidelines, the target level of LDL-C in the group of patients at very high risk is ?1,4 mmol/l and its reduction by 50% from the initial value. However, on high-intensity statin monotherapy, only 32% of patients reach the required range. In this regard, it is extremely important to introduce into clinical practice effective drug technologies for the intensification of lipid-lowering therapy, which ensure high compliance of patients. The appointment of highintensity statin therapy for ACS as early as possible (1-4 days from the onset of the disease) is a proven reasonable approach to improve the prognosis of these patients. In such patients, it is advisable to discuss the appointment of dual and triple combination therapy at an earlier date. The IMPROVE-IT and PRECISEIVUS studies have shown that ezetimibe can be used as a second-line drug in combination with statins [20-22].
The results obtained in the ODYSSEY OUTCOMES study show the benefit of initiating therapy with PCSK9 in the first 3 months after ACS. PACMANAMI study showed that intensive therapy with statins, as well as with PCSK9, slows down the progression of coronary atheroma and can beneficially affect the composition of the plaque by reducing the lipid content in the plaque and increasing the fibrous cap thickness [10][21][23][24].
Initiation of PCSK9 inhibitor therapy according to current clinical guidelines is appropriate when a dual lipid-lowering therapy (high-dose statin + ezetimibe) is ineffective for 4-6 weeks, as well as in initially severe lipid metabolism disorders and a very high cardiovascular risk (LDL-C >4,9 mmol/l) [24].
Since individual biomarkers do not discriminate sufficiently to influence clinical decision making, a multi-marker approach is needed to improve the diagnosis of atherosclerotic plaque vulnerability. The problem with biomarkers is that they often influence each other, i.e. if the level of one of them rises, then it is possible to increase the others.
In assessing plaque vulnerability according to MSCT, the following parameters can be used: an increase in plaque volume, leading to a relative expansion of coronary artery diameter — positive remodeling; low-density area in the plaque (<30 HU*); point calcifications in the plaque composition; an annular enhancement of X-ray density along the plaque periphery, not exceeding 130 HU, an uneven plaque contour or the presence of a gap, but a single interpretation has not yet been proposed [15].
Comparison of paraclinical research methods (OCT, MSCT, CAVI) and a biomarker panel will allow monitoring the progression of coronary atherosclerosis, optimizing methods for preventing cardiovascular risks, and adjusting optimal therapy in patients with acute coronary artery disease [18][19].
Conclusion
The study will allow for the first time to compare and evaluate the effect of PCSK9 inhibitors and ezetimibe in combination with high-dose statin therapy on reducing the manifestations of atherosclerotic plaque vulnerability according to MSCT in non-IRAs in patients with ACS undergoing PCI, as well as to evaluate the diagnostic value of inflammatory biomarkers (GAL-3, hs-CRP, NLR, NGAL) and matrix remodeling (MMP-9, TIMP-1).
Relationships and Activities: none.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Kovalskaya A.N., Bikbaeva G.R., Duplyakov D.V. Effect of combined lipid-lowering therapy on atherosclerotic plaque vulnerability in patients with acute coronary syndrome (Combi-LLT ACS): randomized trial protocol. Russian Journal of Cardiology. 2022;27(4S):5282. https://doi.org/10.15829/1560-4071-2022-5282
Copy