Antihypertensive therapy: controlling the processes of replicative cell senescence
According to the World Health Organization, cardiovascular disease (CVD) is the leading cause of death in developed countries. About 1 billion people worldwide suffer from hypertension (HTN), which is a chronic, slowly progressive disease associated with approximately 7,1 million deaths each year. Clinical studies show a significant relationship between aging and increased blood pressure (BP). Old age is the main nonmodifiable risk factor (RF) for HTN. Therefore, it is necessary to study the indicators of aging. Telomeres are specialized nucleopro- teins that cap and protect the ends of eukaryotic chromosomes, and telomerase maintains telomere length. Together, they play an important role in maintaining the stability of the chromosome ends. Recent data indicate that changes in telomerase activity (TA) and telomere length may be involved in the pathogenesis of HTN. The study of telomere length indicators, TA, the number and aging of circulating endothelial progenitor cells, which are considered markers of vascular aging, provide valuable information on the pathogenesis of age-related conditions such as HTN.
Rationale. Epidemiological studies have shown that the incidence and prevalence of diseases such as coronary artery disease, heart failure, HTN, stroke, increase sharply with age, which is considered the main irreversible RF for CVD. As the world’s population is constantly aging and the proportion of people over 65 will reach approximately 1 billion by 2050, the annual rate of CVD is expected to rise even more. A better understanding of the aging pathways underlying the pathogenesis of HTN will help improve both the diagnosis and selection of antihypertensive agents, which will lead to a decrease in its prevalence.
The aim was to analyze studies over the past two decades on the clinical and biological significance of telomeres as a biological indicator of aging in HTN. The main goal of this review was to study the geroprotective effects of antihypertensives.
HTN as a most important factor in vascular aging
Aging is associated with functional, structural, and mechanical changes in the arteries, which are very similar to vascular changes in HTN. Endothelial dysfunction, vascular remodeling, inflammation, calcification, and increased stiffness are characteristic changes in arteries that develop with age and hypertension. At the cellular level, endothelial cell damage, an increase in vascular smooth muscle cell growth, migration of inflammatory cells, deposition of the extracellular matrix, fibrosis, and calcification are observed [1]. In young patients with elevated BP, arterial changes are observed, similar to those in older people with normal BP, which indicates a premature aging of blood vessels in HTN [2]. Hypertension accelerates age-related changes in blood vessels, which slow down with normalization of BP. A direct association between aging and vascular health is observed in progeria, where patients had accelerated aging, endothelial dysfunction, accelerated atherosclerosis, and premature death from cardiovascular events such as stroke and myocardial infarction [3]. The molecular and cellular mechanisms underlying vascular changes during aging and HTN are common and include aberrant signal transduction, oxidative stress, and activation of pro-inflammatory and pro-fibrotic transcription factors [1].
Effects of oxidative stress, chronic inflammation and insulin resistance on vascular aging
Oxidative stress is common to many of the molecular and cellular processes described above that underlie vascular changes. The idea that reactive oxygen species (ROS) are associated with aging was proposed by Harman in 1956 when he developed the free radical theory of aging. He believed that the accumulation of free radicals during aging damages biomolecules and causes disorders that contribute to the aging of cells and the body. Excessive production of ROS and reactive nitrogen species leads to oxidative modification of proteins, deoxyribonucleic acid (DNA), and lipids, which accumulate in cells and lead to cellular and vascular dysfunction. In addition, the study of the aorta of aged rodents revealed an increase in the vascular level of ROS, a decrease in nitric oxide concentration, which disrupts its vaso- dilatory effect and promotes the formation of a powerful oxidising agent — peroxynitrite [4]. Oxidative stress plays an important role in many molecular processes of vascular aging, including:
- enhancement of pro-inflammatory responses in vascular cells;
- vascular dysfunction through oxidative modification of structural and functional proteins that regulate vascular smooth muscle contraction and relaxation;
- changing calcium homeostasis in vascular cells;
- activation of molecular pathways leading to aging and autophagy in endothelial cells and vascular smooth muscles.
Changes in cellular antioxidant systems are also important because the expression and activity of antioxidant enzymes in tissues, including superoxide dismutase, decrease with age. A decrease in antioxidant efficiency is also facilitated by the suppression of nuclear factor erythroid 2-related factor 2 (NRF2) which is the main transcription factor that regulates antioxidant genes [5]. Numerous oxidases generate ROS in the vascular wall and endothelium, including NADPH oxidase (NOX), xanthine oxidase, and mitochondrial oxidases. There are 7 NOX isoforms (NOX 1-5, DUOX1, DUOX2) that contribute to oxidative stress during vascular aging. In particular, in aged rats with spontaneous HN in the aorta, the expression of NOX1 and NOX2 was increased. This NOX activation was associated with endothelial dysfunction, which was reversed by the NOX inhibitor VAS2870. NOX appears to be important in pathological vascular remodeling associated with HTN and CVD. Vascular xanthine oxidase and cytochrome P450 epoxygenase are apparently less important, since the expression and activity of these systems do not change with aging in humans [6].
During biological aging, mitochondrial dysfunction occurs, resulting in decreased energy production and increased ROS synthesis. Mechanisms associated with mitochondrial dysfunction during aging include decreased adenosine triphosphate synthesis, increased apoptosis, and mitochondrial DNA mutations during oxidation. During aging, mitochondrial electron flow decreases, altering oxygen consumption and causing the ROS production. The pro-oxidative effects damage mitochondrial DNA, leading to further dysfunction of the electron transport chain and increased ROS production. Consequently, the apoptosis rate increases, releasing an excess amount of ROS into the cytosol, which further contributes to oxidative stress and damage to vascular cells [7].
With aging, there is a shift towards a pro- inflammatory vascular phenotype with the activation of inflammatory cytokines, chemokines, and adhesion molecules in the vessel wall. Pro-inflammatory transcription factors and proteins that have been identified in aging vessels include: monocyte chemoattractant protein-1 (MCP-1) is one of the key chemokines that regulate migration and infiltration of monocytes; transforming growth factor ?1 (TGF-?!) regulates proliferation, cell differentiation, and other functions in most cells and is involved in the immune response; matrix metallo- proteinase 2 (MMP-2) plays a role in tissue remodeling, angiogenesis, proliferation, cell migration and differentiation, and apoptosis. Activator protein 1 (AP-1) is a transcription factor that regulates a number of cellular processes, including differentiation, proliferation, and apoptosis; nuclear factor kappa- light-chain-enhancer of activated B cells (NF-kB) is a universal transcription factor that regulates the expression of genes of the immune response, apopto- sis, and the cell cycle. The expression and activation of these molecules increases with aging, and these processes are also associated with increased ROS production. In aged arteries, a decreased expression of the transcription factor NRF2 is observed, which stimulates the expression of antioxidant enzymes, thereby leading to a decrease in the antioxidant potential and increased bioavailability of RO S, followed by oxidative stress. Oxidative stress is a powerful inducer of proinflammatory signaling pathways, contributing to further inflammation and vascular damage during aging [8].
Also, insulin resistance (IR) plays an important role in the vascular aging, which promotes the activation of oxidative stress and an increase in chronic inflammation. Thus, IR reduces the number of endothelial progenitor cells, which reduces reparative activity and contributes to the degeneration.
Hyperinsulinemia enhances the synthesis of very-low-density lipoproteins, increases the transfer of cholesterol to arterial smooth muscle cells (SMC), stimulates their proliferation, enhances collagen synthesis, and activates genes involved in inflammation. IR is also associated with decreased NO synthesis and increased ROS production. The release of free fatty acids from adipose tissue also increases, which disrupts endothelial function and induce chronic inflammation [9].
Treatment of HTN as a way to reverse vascular aging
The diagnosis of age-related vascular changes is based on the measurement of the ankle-brachial index (peripheral arterial disease), pulse wave velocity (arterial stiffness), carotid intima-media thickness (atherosclerosis), and arterial dilatation (endothelial dysfunction). Arterial stiffness is a marker of CVD. Accelerated vascular aging is an increase in arterial stiffness that does not correspond to the current chronological age Treatment of early vascular aging is important for the primary prevention of CVD. There are the following factors that slow the progression of vascular aging: increased physical activity, controlled drinking, reduced salt intake, and weight loss. HTN, diabetes, obstructive sleep apnea and dyslipidemia are factors that accelerate vascular damage and should be treated and monitored for a long time [10].
Hyperactivity of the renin-angiotensin-aldo- sterone system (RAAS) increases the risk of CVD. Overstimulation of angiotensin II receptors type 1 (AT1) and mineralocorticoid receptors leads to cell growth, vascular inflammation, and oxidative stress, which leads to arterial stiffness and acceleration of vascular aging [11]. Thus, AT1 block during the hypertension treatment prevents the effects of angiotensin II (Ang II) mediated by these receptors, which prevents the adverse effect of Ang II on vascular tone and is accompanied by BP decrease, contributing to vasodilation, natriuresis, and a decrease in collagen deposition, thus decreasing arterial stiffness and improving endothelial function.
In a study by Statsenko ME, et al. with 30 patients aged 40-70 years with stage 2-3 HTN and type 2 diabetes, the effects of antihypertensive therapy on large vessels were studied. The patients received therapy with enalapril and indapamide. The arterial elasticity was analyzed by measuring carotid-femoral pulse wave velocity (PWV) of elastic and muscular arteries. After 12 weeks of therapy, there was a significant decrease in carotid-femoral PWV of elastic arteries by 10,8% and muscular arteries by 10,1% (p<0,05). This treatment significantly reduces the vascular stiffness of large arteries [12]. A study by Liventseva MM, et al. with 40 patients with grade 2-3 HTN aged 18-65 years also showed a decrease in arterial stiffness with 3-month use of a fixed-dose combination of an angiotensin-converting enzyme (ACE) inhibitor lisinopril and a calcium antagonist amlodipine. Increased arterial stiffness is associated with endothelial dysfunction, and amlodipine expresses an antihypertensive effect on the endothelium, which improves its function. After 3 months of taking the drug, PWV decreased from 10,1±0,3 m/s to 8,1±0,2 m/s (p<0,001) [13].
We can conclude that the HTN treatment has a positive effect on the arterial walls, i.e., it slows down vascular aging.
Effect on telomere biology as a possible way to slow down the aging
Since aging is the main RF of HTN, it is necessary to study aging indicators. Telomere length is one of these indicators, which depends on age and TA. A relationship has been established between a decrease in telomere length and an increase in the risk of many diseases, including the CVD.
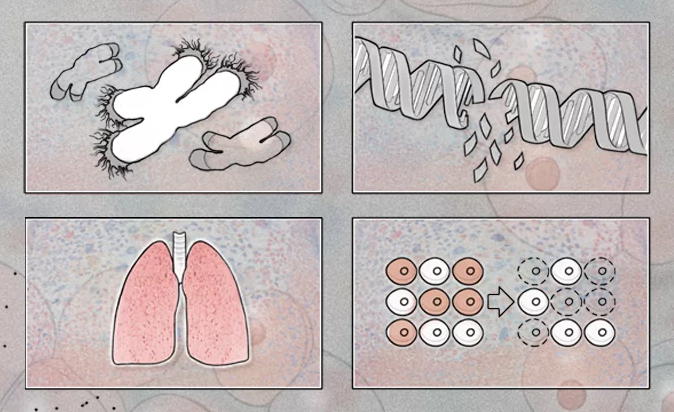
Telomeres are regions of specific telomeric DNA and proteins at each end of a chromosome. Functional telomeres are the main mechanism for preventing the cellular DNA damage response resulting from the recombination and degradation of chromosomes [14]. With each mitosis, telomeres shorten, which leads to the inability of cells to divide endlessly and, accordingly, causes natural aging of the body. When the critical length, or the so-called Hayflick limit, is reached, telomeres cannot perform their functions. The cell cycle is disrupted, then the cell dies, which leads to aging and death of the body. There are following main functions of telomeres: providing the chromosome-nuclear envelope fixation; facilitating homologous recombination in meiosis; maintaining the genome integrity; ensuring the stabilization of damaged ends of chromosomes; regulation of gene expression; determination of the replicative potential of the cell [15]. It is assumed that in HTN pathogenesis, a decrease in telomere length and TA is important. Telomerase is an enzyme that adds specific repeated DNA sequences to the 3’ end of the DNA at regions of telomeres. Functionally, telomerase is a special reverse transcriptase that works together with a special telomerase RNA. Telomerase-associated proteins include the telome- rase components TERC and TERT. This enzyme is synthesized in the cytoplasm; TERC is expressed in all cells, and TERT — in gametes and transformed cells [16]. Sex and stem cells have the highest TA, while somatic cells of an adult have a low activity of this enzyme [17].
The research results indicate an inverse correlation of telomere length with pulse pressure (PP) and PWV [18]. HTN is a hemodynamic disorder, and an increase in vascular stiffness, PP, and PWV is the distinctive hemodynamic features of an increased BP. Consequently, the factors influencing the risk of CVD are the values of PP and carotid-femoral PWV.
Study by Jeanclos E, et al. included 98 healthy twins aged 18-44. It was found that the PP values correlate inversely with the telomere length in leukocytes. Telomere length and PP were hereditary in nature; the revealed relationship did not depend on sex [19].
Balistreri CR, et al. studied biomarkers of vascular aging, including the red blood cell distribution width (RDW), telomere length, TA, and endothelial progenitor cells (EPC). For this, 80 elderly (72±4,8 years) and 80 young (26,2±3,4 years) healthy patients and 80 patients with CVD were selected. The main results obtained showed that increased values of RDW and high-sensitivity C-reactive protein in the blood and decreased mean values of telomere length in leukocytes, TA and EPC are independently associated with a high risk of vascular aging [20].
The study by McDonnell BJ, et al. included 904 patients, who were divided into two age groups (<30 and >50 years of age) with an equal sex ratio. This study showed that in the younger group, telomere length was significantly shorter in patients with high levels of aortic PWV compared with patients with low levels (p=0,017). In contrast, in the elderly, telomere length was significantly longer in patients with high aortic PWV (p=0,001). Age significantly changed the relationship between aortic PWV and telomere length (p<0,001) [21].
Current studies on telomere length may indicate that short telomeres are the RF of CVD, in part via insulin-mediated pathways. However, further studies with quantification methods and larger patient cohorts are needed to clarify the additional role of telomere length in predicting CVD risks [22].
Effect of RAAS blockers on telomere biology
RAAS is a complex hormonal-enzymatic system, the impairment of which plays a decisive role in the development of HTN. The effects of the main RAAS participants are varied and are not limited to an increase in total peripheral resistance and the retention of sodium and water. Angiotensin receptors are widely represented in almost all organs and tissues, mediating the many-sided effect of the RAAS on body physiology. In a number of studies, relationship of RAAS with aging was proved.
This relationship was first demonstrated by Benigni A, et al. (2009), who showed that targeted blocking of genes encoding AT1 receptors increases lifetime in mice. The population of such mice had lower number of CVDs, and also less pronounced oxidative stress in organs and tissues [23]. Subsequently, numerous studies have identified the possible directions of RAAS effects on vascular aging: activation of oxidative stress in vascular SMC, causing chronic replicative aging [24][25]; influence on the sirtuin biology [26][27][28][29]; influence on the mammalian target of rapamycin (mTOR) [30] and others. In clinical practice, the main RAAS targets of pharmacological action are ACE (ACE inhibitors) and AT1 (AT1-receptor blockers). A possible advantage of an ACE inhibitor over AT1-receptor blockers is an increase in the bradykinin levels in tissues. A number of studies have shown that bradykinin slows down cell aging by regulating the redox state in tissues, but its isolated effect on telomere biology has not yet been considered [31][32].
Effect of ACE inhibitors on telomere biology
Studies on the effect of ACE inhibitors on telomere biology were carried out both ex vivo and in vivo.
Ex vivo studies have demonstrated the positive effect of RAAS blockers on telomere biology, including the study of AT1-receptor blockers: valsartan and losartan [33][34][35]. In addition, an experimental ex vivo study by Donnini S, et al. was conducted on the effect of various ACE inhibitors on the endothelial cell culture of bovine postcapillary vessels, cultured in serum with an extremely low concentration (0,1%) and devoid of nutrients. Thus, the culture conditions were as close as possible to those of oxidative stress in CAD. The functional parameters of the endothelium (survival, angiogenesis), as well as markers of apoptosis, aging and cell division (AKT, NO/c-GMP, FGF-2, TERT, and caspase-3) were assessed under exposure to ACE inhibitors with sulfhydryl (SH) group (captopril, zofenoprilat) and without (enalaprilat, lisinopril). According to the study, ACE inhibitors containing SH group showed more pronounced protective properties in relation to endothelial cells, and zofenoprilat showed the greatest efficiency. In terms of telomere biology, zofenoprilat was also successful, increasing the expression of TERT matrix ribonucleic acid (mRNA) 6-fold in the studied culture. Enalapril had the lowest efficiency in expression among all drugs. Most likely, zofenoprilat induces TERT mRNA expression through the activation of fibroblast growth factor 2 (FGF-2) genes. It was shown that the silencing of FGF-2 genes upon exposure to zofenoprilat does not increase in TERT mRNA in response to the drug, which suggests a causal relationship between these events [36].
Perindopril is also of great scientific interest. There have been a lot of studies proving the pleio- tropic effect of perindopril, including on indicators directly or indirectly associated with telomere dysfunction. The positive effect of perindopril on vascular aging has been demonstrated in the studies PERSPECTIVE (PERindopriTS Prospective Effect on Coronary a Therosclerosis by angiography and IntraVascular ultrasound Evaluation) and DAPH- NET (Diabetes Artery Perindopril Hypertension Normalization Excess sTiffness). DAPHNET study showed that 6-month therapy with perindopril at a dose of 8 mg/day reduces vascular stiffness in patients with HTN and diabetes [37]. The analysis of the PERSPECTIVE study demonstrated that perindopril can reverse noncalcified atherosclerotic plaques [38].
The study of perindopril effect on telomere biology, namely, on TA, was carried out in an open- label comparative randomized study by Strazhesko ID (2019). The study initially enrolled 52 patients with diagnosed HTN. Participants were equally randomized to the perindopril group and to the group of other antihypertensives, with the exception of drugs affecting the RAAS. During the year, 11 people withdraw from the study for various reasons. Thus, 24 patients remained in the perindopril group and 17 people in the group with other antihypertensives. According to the study, treatment with perindopril did not lead to a significant change in TA. Nevertheless, vascular aging parameters were improved: vascular elasticity (decrease in PWV by 9,5%, p=0,035), decrease in intima-media thickness (by 7,9%, p=0,034). The absence of a significant effect on telomere biology can be associated with the absence of RAAS hyperactivation in target organs and, as a consequence, the absence of chronic inflammation and oxidative stress. More reliable results require a larger randomized trial [39].
Effect of angiotensin receptor blockers
Ang II modifies cell proliferation by inducing TGF-?! and direct modulation of the G1 phase by activating cyclins and cyclin-dependent kinases; as a complex, they lead to the phosphorylation of retinoblastoma protein, which inhibits the cell cycle by creating a “point of no return” in the G1 phase.
Chronic inhibition of ACE, regardless of BP, reduces vascular damage during aging. This emphasizes the role of the RAAS as a potential mediator in accelerated cell metabolism in young spontaneously hypertensive rats. This idea is supported by the observation that the initial increase in cell proliferation in spontaneously hypertensive rats coincides with the time window of Ang II hyperactivation [40]. In addition, RAAS inhibition during this time window leads to protection of the cardiovascular system up to old age without a long-term decrease in BP [41].
In the study by Baumann M, et al. [41], one group of spontaneously hypertensive rats were treated with losartan and another group was managed without therapy. Data control was performed at 8 and 72 weeks of life. In young rats, systolic BP was significantly reduced in comparison with rats not receiving therapy (P<0,05). Plasma TGF-?! levels were assessed as a marker of proliferation and were highest in rats not receiving therapy. Losartan significantly reduced plasma TGF-?! levels to normal values. The telomere length also differed in rats receiving and not receiving losartan (without therapy 1,0±0,1 kb, losartan 2,8±0,3 kb, P<0,01). Moreover, oxidative damage was reduced in the losartan group over the entire follow-up period.
It can be concluded that increased proliferation in HTN leads to increased cell turnover, subsequently leads to cellular aging, which is determined by a decrease in telomere length. Thus, in rats treated with losartan, proliferation decreases due to transient antagonism of Ang II receptors, which is associated with longer telomeres and indicates a slowdown of aging [40].
The study by Wang L, et al. investigated the change in telomere length in the culture of human renal mesangial cells during aging induced by Ang II, and the effects of losartan on them. As an angiotensin II receptor blocker, losartan blocks the binding of Ang II and its receptor. Cell cycle analysis showed that the Ang II + losartan group had a significantly lower cell ratio in the G0/G1 phase and a higher cell ratio in the S and G2/M phase compared to the Ang II group. These changes indicate that losartan delays Ang II-induced cellular senescence in SMC. The Ang II + losartan group showed a significantly greater telomere length (3,99±0,066 kb) than in the Ang II group (3,03±0,096 kb), but still shorter than in the control group. These changes confirm that Ang II induces telomere length shortening in SMC.
Thus, these studies confirm that Ang II is an additional factor accelerating telomere shortening related to aging. Losartan reduces Ang II-induced telomere shortening in mesangial cells, thereby slowing cell aging [34].
The study by Zhou H, et al. demonstrated similar results: Ang II induces the aging of SMC in human mesangial cells. This process involves the JAK2/ STAT signaling pathway that is a chain of interactions between proteins in a cell, where JAK is a Janus kinase, non-receptor tyrosine kinases, and STAT is a signal transducer and activator of transcription (STAT) protein family, which are intracellular transcription factors that provide many aspects of cellular immunity, proliferation, apoptosis, and differentiation. Using losartan and blocking the JAK2/ STAT pathway, it is possible to delay the aging of SMC [42].
The study by Kobayashi K, et al. investigated the effect of Ang II on the differentiation and aging of bone marrow-derived endothelial progenitor cells (BM-EPC). The ability of EPC to participate in endothelial repair is impaired by Ang II and other atherogenic factors. The Wistar rats (n=40) were injected with Ang II alone or in combination with an angiotensin II receptor blocker (valsartan). The rats in the valsartan group had significantly fewer differentiated adhesive BM-EPCs than the control group. The addition of valsartan restored the level of attached differentiated BM-EPCs to the control group level. The number of aging BM-EPCs was significantly higher in the group of Ang II only than in the valsartan one. Enzyme-linked immunosorbent assay (ELISA) showed that TA was significantly lower in BM-EPC from the group of Ang II only, and the addition of valsartan significantly increased its activity. The analysis showed that Ang II significantly reduced functional activity in BM- EPCs, and this effect was significantly reduced by valsartan [43].
Effect of other antihypertensives on telomere biology
There are very few studies on the effect of non- RAAS inhibitor antihypertensives on telomere biology. Moxonidine is a promising drug for reducing cellular aging. Some studies showed that moxonidine can modificate pathological conditions leading to vascular aging, namely, various components of the metabolic syndrome, including BP, obesity, and IR [44][45][46][47].
Dudinskaya EN, et al. studied the effect of moxonidine and bisoprolol, including on TA. Randomized comparative clinical study included 114 postmenopausal women with diagnosed primary HTN and osteopenia/osteoporosis. Although the aim of the study was to assess the effect of mo- xonidine on bone metabolism and bone density in postmenopausal women with HTN and osteopenia, TA was also evaluated. The patients were randomized into two groups: bisoprolol at a dose of 5 mg (n=46) or 7,5 mg (n=11) and moxonidine at a dose of 0,4 mg (n=49) or 0,6 mg (n=8). After a 1-year moxonidine therapy, there was a significant increase in TA from 0,87 to 1,15 (p<0,01), in contrast to the bisoprolol group, where TA, on the contrary, significantly decreased from 0,89 to 0,64 (p=0,01); TA delta in the moxonidine group was 45,46%, while TA delta in the bisoprolol group was 13,99%. During the study, the pleiotropic effect of moxonidine was shown, including an increase in TA. Most likely, the beneficial effect of moxoni- dine on the telomere biol ogy was realized due to a decrease in IR, since therapy with moxonidine resulted in weight loss (initial body mass index: 29,3±4,7, after 12-month therapy: 28,9±3,8) [44]. Obesity in women is one of the reliable causes of oxidative stress, leading to a decrease in telomere length. Overweight and obesity are often associated with IR, and related hyperinsulinemia can affect telomere biology.
Studies on the effect of diuretics on telomere length and TA have not yet been conducted. However, there is evidence that indapamide has a positive effect on the vascular wall. It is proposed that indapamide promotes an increase in prostacyclin synthesis, a decrease in platelet aggregation and thromboxane A2 release, which leads to vasodilation and a decrease in the cardiac load [48].
In the study by Semenkin AA, et al. with 50 hypertensive patients, antihypertensive and metabolic effects of thiazide diuretics (hydrochlorothiazide and indapamide) on vascular endothelial function and cardiovascular risk was studied for 12 weeks. The effect of drugs on endothelium-dependent vasodilation improved with indapamide (+8,9%; p=0,10) and was significantly worse with hydrochlorothiazide (-17,0%; p<0,05) [49].
Conclusion
HTN and conditions such as IR and oxidative stress, directly associated with this disease, are the main causes of vascular aging. Parameters such as PWV, PP, ankle-brachial index, intima-media thickness, and endothelium-dependent vasodilation reflect functional, structural, and mechanical changes in arteries associated with age, but telomere length is the main indicator of biological aging. The interactions between vascular aging and telomere biology require further large-scale studies, but the available results suggest that age-related vascular changes are more pronounced in patients with short telomeres. The question of whether short telomeres are RF for CVD remains unresolved.
Short telomeres are associated with a decrease in cell replicative potential, which entails a decrease in the regenerative abilities of all body systems and underlies aging. At this stage, the description of biological age as nonmodifiable RF of CVD is still relevant, but the results of the studies reviewed show that RAAS blockers hold promise for improving telomere biology. The probable efficacy of the considered drugs as geroprotectors is associated with the block of Ang II hyperactivation pathways, which entails a decrease in the activation of various signaling pathways leading to excessive proliferation of vascular wall cells, and a decrease in ROS production by NADPH oxidase. Large-scale human subject studies are required to confirm the efficacy of this group of drugs. It is possible that other antihypertensive agents are effective in reducing cellular aging, including moxonidine, which has already been shown to be effective in increasing TA in women with primary HTN, but these effects also require confirmation in larger clinical trials.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Dudinskaya E.N., Machekhina L.V., Eruslanova K.A., Dogotar O.A., Ryltseva L.P., Lyzlova N.Yu., Shchepin N.A., Kotovskaya Yu.V., Tkacheva O.N. Antihypertensive therapy: controlling the processes of replicative cell senescence. Russian Journal of Cardiology. 2020;25(3S):3974. https://doi.org/10.15829/1560-4071-2020-3974
Copy