Prevalence of cardiac arrhythmias among patients undergoing chronic hemodialysis
The high prevalence of cardiovascular disease (CVD) among patients undergoing hemodialysis (HD) is a serious problem requiring attention from cardiologists. It has been established that a decrease in glomerular filtration rate is associated with increased incidence of CVD and mortality [1]. This relationship is so strong and clinically significant that, according to current guidelines, the diagnosis of chronic kidney disease (CKD) places the patient at the highest level of cardiovascular risk, regardless of stratification according to traditional risk factors [2, 3]. High mortality in patients on renal replacement therapy, which can be up to 375 times higher than in the general population [4], is almost half due to cardiovascular causes [5].
The term “cardiorenal syndrome” refers to the interaction between the heart and kidneys and includes 5 different types depending on the initial affected area and the acute or chronic nature of the damage [6]. Type 4, or chronic renocardiac syndrome, includes factors associated with CKD that lead to cardiac and vascular disease. Arrhythmias and sudden cardiac death (SCD) account for 26,9% of mortality in patients with stage 5 CKD receiving HD [7].
Although patients with CKD have an increased risk of arrhythmias, the underlying mechanisms and their association with SCD are not fully understood [8][9]. Several explanations have been proposed, but they are likely due to structural cardiac alterations. Patients with stage 5 CKD on HD often have a uremic cardiomyopathy, which includes varying degrees of left ventricular (LV) hypertrophy and dilatation, systolic and diastolic dysfunction, and fibrosis, which predisposes the patient to arrhythmias [10].
Previous studies of arrhythmias among patients receiving HD were conducted mainly in relatively small groups, the number of which does not exceed 50-70 patients, which explains the large scatter of data on the prevalence of arrhythmias in patients undergoing chronic dialysis. Most of the information was obtained from short-term Holter electrocardiogram recordings in the 1980s and 1990s [11][12]. At the same time, we now observe that the population of dialysis patients has changed markedly compared to earlier studies — current patients are older and have many comorbidities, while the proportion of patients with diabetes is also growing [13-15].
The main aim of our study is to assess the prevalence of arrhythmias in patients receiving chronic HD, to characterize the types of arrhythmic events in relation to the dialysis procedure, and to determine their relationship with clinical data and echocardiographic parameters.
Material and methods
The study included 152 people in 2019-2021. All patients signed written informed consent approved by the local ethics committee at the Rostov State Medical University. Included patients received HD for at least 6 months. There were following exclusion criteria: implanted pacemakers, heart transplant, patients with acute myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, cerebrovascular accident, with class III-IV coronary artery disease or NYHA class III-IV heart failure, terminal cancer, residual renal function, inadequate dialysis dose, and presence of a central venous catheter. All patients were observed by a cardiologist at the start of the study, during the recruitment period, and during follow-up. The dialysis procedure was carried out according to the standard method (model, 4008 or 5008; Fresenius Medical Care) using high-flux dialyzers ranging in size from 1,4 to 2,5 m2 .
The dialysate contained potassium 2,0 mmol/l and glucose 1 g/l. The concentration of sodium (average 138±0,9 mmol/l), ionized calcium (87%, 1% and 12% from 1,25, 1,50 and 1,75 mmol/l, respectively) and bicarbonate (35,3±2,4 mmol/l).
All patients underwent history taking, measurement of weight, height, blood pressure (BP) (average of the last 2 of 3 assessments), evaluation of lipid status, biochemical parameters, plasma electrolytes, as well as 48-hour ECG monitoring, which started before the dialysis session.
The criterion for establishing the diagnosis of atrial fibrillation/flutter (AF/AFL) was the presence of a recorded episode of arrhythmia with characteristic ECG signs lasting no less than 30 seconds, in the presence of an episode of supraventricular tachycardia lasting >30 seconds with characteristic ECG signs of AF/AFL. Supraventricular tachycardia was considered as ?3 consecutive heart beats with a frequency above 100 bpm, where sinus node, atrial myocardium and/or atrioventricular (AV) node are involved in the mechanisms of occurrence and self-maintenance of arrhythmia [16]. Supraventricular tachycardia (SVT) was ?5 ectopic supraventricular beats with heart rate (HR) >100 bpm, without AF [17].
HR values during Holter ECG, referred to bradycardia, were <40 bpm for ?10 sec. There were following criteria for sinus node dysfunction: continuous sinus bradycardia for 24 hours with heart rate <50 bpm; pauses >3 sec; persistent or intermittent periods of symptomatic AV escape rhythm; documented brady-tachycardia syndrome. Nonsustained ventricular tachycardia (VT) was established when registering ?3 ventricular contractions with a heart rate ?100 bpm. Supraventricular premature beats (SPBs) were considered pathological if the number was >200 per day or >30 per hour. Premature ventricular contractions (PVCs) were classified according to Lown and Wolf classification [18].
Arrhythmias were summarized as follows: bradyarrhythmias — bradycardia, sinoatrial, and secondor third-degree AV block; tachyarrhythmias: paroxysmal AF, paroxysmal SVT, and nonsustained VT.
ECG monitoring was carried out using cardiac monitor devices (PADSY, Germany). The recording was made within 48 hours.
Echocardiography was performed on 93 patients prior to dialysis on Phillips system using a convex transducer by a specialist and were recorded for later review. Ultrasound included measurements of LV mass index, Simpson’s LV ejection fraction, left atrial (LA) volume index, E/e’, cardiac output, and preload. Also, increased preload was defined as increased LV filling pressure (E/e’ >12) and an increase in LA (LA volume index >30 ml/m2).
Statistical data processing. Data were expressed as mean ± standard deviation for normally distributed data and median with interquartile range [IQR] for non-normally distributed data. Categorical variables were expressed as frequency and percentage. Comparisons between groups were performed using the t-test or one-way analysis of variance for normally distributed data and the Wilcoxon rank sum test or Kruskal-Wallis test for non-normally distributed vaiables. For categorical variables, comparisons were made using ?2 or Fisher’s exact test when appropriate. Paired data comparison was performed using the paired t-test for normally distributed data and Wilcoxon’s signed rank test for non-normally distributed data. The records were divided into 6 periods of 8 hours each. The first period included a dialysis session and the period after it. A multivariate logistic regression model was used to subanalyze echocardiographic data associated with arrhythmias. P<0,05 was considered significant. All statistical studies were performed using Stata version 14 (StataCorp, 2015).
Results
A total of 152 patients undergoing chronic HD were included in the study. Patient data are presented in Table 1. Initially, 42 (27,6%) patients had a history of palpitations. During recording, only 5 (3,3%) patients felt palpitations, of which only 1 case was associated with a clinically significant arrhythmia (AF with high ventricular rate). Also, during the Holter ECG recording, 5 (3,3%) patients experienced a chest pain and 2 (1,3%) had syncope of non-cardiac origin, while 5 patients (3,3%) had symptomatic intradialytic hypotension. However, none of these conditions was accompanied by clinically significant arrhythmia.
Prevalence of arrhythmias
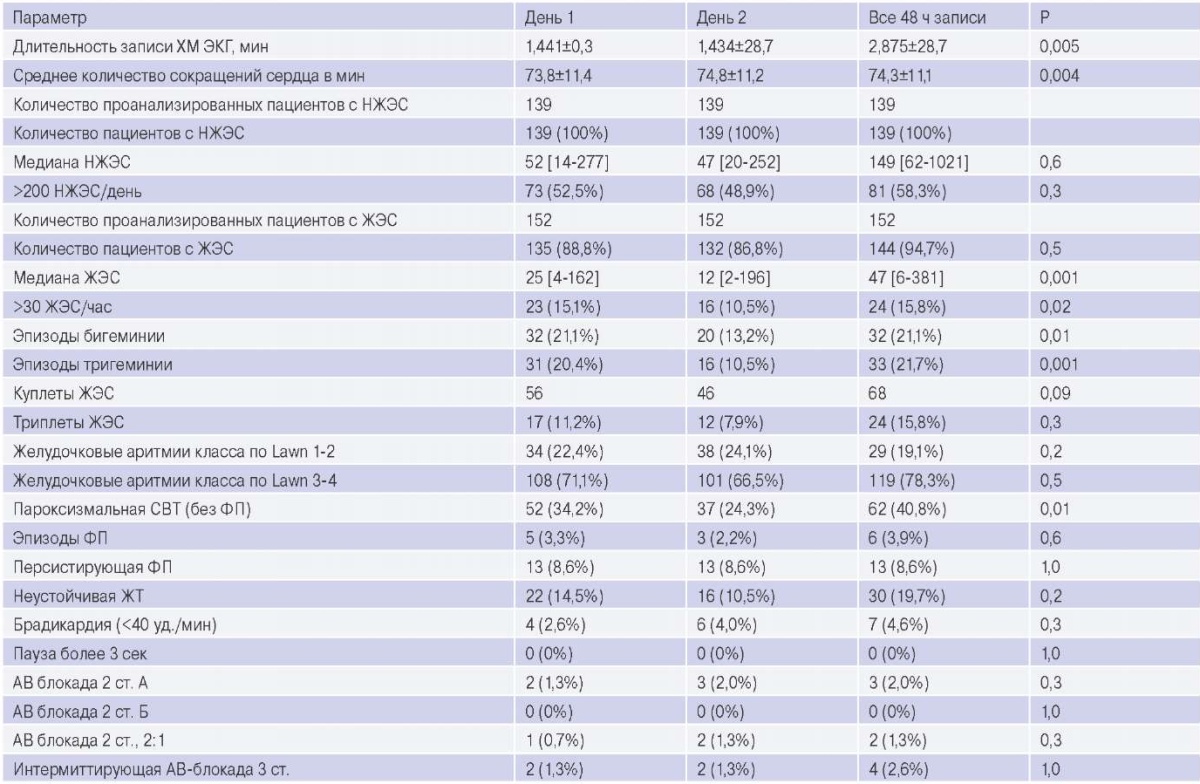
Data on the prevalence of arrhythmias in the study group are presented in Table 2.
SPBs and PVCs were observed in almost all patients. The mean number of PVCs was higher in patients on the day of dialysis (Day 1) compared to the day without dialysis (25 [ 4-162] vs 12 [ 2-196], p<0,001). Complex ventricular arrhythmias (Lown class III-V) were observed in 119 (78,3%) patients. Permanent AF was present in 13 (8,6%) patients, and AF episodes were found in 6 (3,9%) patients. The number of AF episodes ranged from 1 to 14 per day, with a maximum duration of each episode ranging from 37 to 861 minutes in individual patients. In 5 patients, the first recorded AF episode occurred during a dialysis session. A greater number of VT episodes were registered on the dialysis day. Asymptomatic bradyarrhythmias were observed in 10 patients. Two patients had episodes of third-degree AV block during dialysis and 2 patients at night. In the course of the study, due to bradyarrhythmias, no indications for implantation of a pacemaker were found in any of the patients. A total of 65 patients had 549 tachyarrhythmia episodes and 8 patients had 3204 bradyarrhythmia episodes. Tachyarrhythmias were significantly more common in the 8-hour interval, including dialysis and the period immediately after dialysis. A total of 3 patients showed a trend towards an increase in bradyarrhythmia at the end of the interdialytic interval.
There were following factors associated with arrhythmia: advanced age (odds ratio (OR) of 10 years, 1,53; 95% confidence interval (CI): 1,15-2,03; P=0,003), longer period of dialysis (OR of 1 year, 1,11; 95% CI: 1,02-1,21; P=0,02), lower systolic BP (OR of 1 mm Hg, 0,97; 95% CI: 0,95-0,99; P=0,01), history of palpitations (OR, 2,45; 95% CI: 1,06-5,68; P=0,04). In addition, SPBs >200 per day were correlated with an increased incidence of AF episodes (7,4% vs 0%; P=0,04) and with a history of paroxysmal AF (34,6% vs 6,9%; P<0,001). Among dialysis patients, lower post-dialysis plasma ionized calcium (OR of 0,1 mEq/L higher, 0,27; P=0,02) was associated with dialysis-day AF episodes in univariate analysis. We did not find independent predictors of bradyarrhythmias in regression models, but all patients with bradyarrhythmia were male (10,8% vs 0% (female); P=0,02).
Echocardiographic data and arrhythmias
Echocardiographic predictors of arrhythmias were investigated in a separate analysis in patients using multivariate analysis (Table 3).
A higher LV mass index was associated with AF in multivariate models (OR with LV mass index greater by 1 g/m2, 1,02; (95% CI: 1,00-1,04; P=0,04) and low cardiac output (OR with cardiac output greater by 1 L/min, 0,54; (95% CI: 0,28-1,04; P=0,06). An association was found between ventricular arrhythmias — frequent PVCs with paroxysms of VT with reduced cardiac output and increased preload, defined as increased LV filling pressure and increased LA volume Bradyarrhythmia, VVP, and paroxysmal SVT were not predictable by echocardiographic data in multivariate models.
Table 1
Characteristics of patients
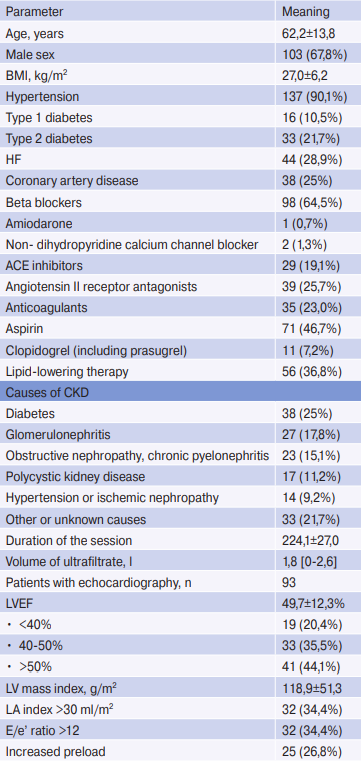
Note: n=152. Values for continuous variables are given as mean ± standard deviation or median [interquartile range]; for categorical variables as number of patients (percentage).
Abbreviations: ACE — angiotensin-converting enzyme, HD — hemodialysis, CAD — coronary artery disease, BMI — body mass index, LV — left ventricle, LA — left atrium, EF — ejection fraction, CKD — chronic kidney disease, HF — heart failure.
Table 2
Registered arrhythmias during the 48-hour Holter monitoring

Note: n=152. Values for continuous variables are given as mean ± standard deviation or median [interquartile range]; for categorical variables as number of patients (percentage). The table shows the number of episodes of arrhythmias and the percentage of patients from the total group in whom these episodes were registered.
Abbreviations: AV block — atrioventricular block, VT — ventricular tachycardia, PVC — premature ventricular contractions, SPBs — supraventricular premature beats, SVT — supraventricular tachycardia, AF — atrial fibrillation, HM ECG — electrocardiographic Holter monitoring.
Table 3
Multivariate analysis of associations between echocardiographic parameters and arrhythmias
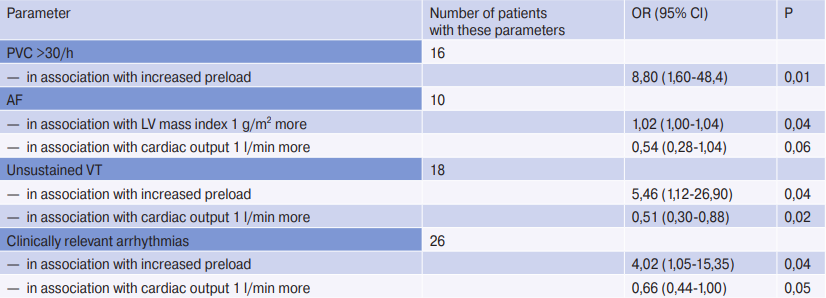
Note: variables were selected by automatic forward sampling from the following covariates: LV ejection fraction, LV mass index, LA volume index, LA volume index >30, E/e’ >12, increased preload, and cardiac output. Analysis of atrioventricular and paroxysmal SVT were performed in patients without permanent AF (n=90). All variables included in the final models are listed below each result in the first column. Increased preload was defined as E/e’ >12 and LA volume index >30 ml/m2. Clinically relevant arrhythmias were considered AF, non-sustained VT, bradycardia, pauses >3,0 sec, and 2- or 3-degree atrioventricular block.
Abbreviations: CI — confidence interval, VT — ventricular tachycardia, PVC — premature ventricular contractions, LV — left ventricle, LA — left atrium, OR — odds ratio, SVT — supraventricular tachycardia, AF — atrial fibrillation.
Discussion
In the study of 152 patients receiving chronic dialysis, a high incidence of both non-life-threatening and clinically significant arrhythmias was demonstrated. Tachyarrhythmias were more common during dialysis and immediately after it. All clinically significant arrhythmias, except for a single episode, were asymptomatic. Arrhythmias was associated with such variables as: older age, longer period of dialysis, lower systolic BP, history of palpitations, lower plasma ionized calcium before dialysis, higher LV mass index, increased preload, and low cardiac output. According to the literature data, the prevalence of AF in this group of patients is from 5% to 27% in patients, based on data from registries and studies using short-term records; also according to these data, AF is often asymptomatic, and the prevalence is most likely underestimated [19] . According to our data, all patients had SPBs, but it is noteworthy that patients with SPBs (>200 per day) had a higher risk of AF episodes, which is consistent with the literature data. We identified only 2 patients with newly diagnosed AF. In 35 (81%) patients with previously documented AF paroxysms, no signs of AF were found during the recording. Buiten MS, et al. reported that AF episodes were more common on dialysis days and especially during the dialysis session, which coincided with our data [20]. According to the literature, PVCs are often considered benign, but the presence of PVCs >30 per hour is often associated with a higher risk of death and SCD in the general population [21]. In our study, there was a higher risk of nonsustained VT in patients with >30 PVCs per hour. According to the literature data, PVCs (grades III-V according to Lown and Wolf) were observed in 0-61% of patients, which is lower than in our results (78,3%). Alfraihat N, et al. report episodes of bradycardia in 30% of patients, synatrial block in 28%, and second-degree AV block in 4% of 50 patients during long-term follow-up (18±4 months) [22]. In addition, in studies of implantable loop recorders, bradyarrhythmia was the most common terminal arrhythmia in cases of SCD [17]. Asymptomatic bradyarrhythmias occurred only in 10 (6,5%) patients, which may be due to the short follow-up time. We observed more tachyarrhythmias during dialysis and immediately after dialysis compared to the rest of the observation period. Also, more PVCs and more episodes of ventricular bigeminy or trigeminy were recorded on the day of dialysis, which is comparable to the literature data, in which more PVCs were found during dialysis and in the post-dialysis hours [23]. These data support the hypothesis of dialysis as a trigger for arrhythmias.
We have identified several clinical characteristics independently associated with arrhythmias. Older age was positively associated with SPBs >200 per day, paroxysmal SVT, >30 PVCs per hour, and the presence of clinically significant arrhythmias. The period of the dialysis procedure was positively associated with patients having >30 PVCs per hour. A history of palpitations was independently associated only with SVT paroxysms. Lower systolic BP was associated with AF, which could be explained by the presence of heart failure in these patients. Surprisingly, diabetes was associated with less frequent SPBs and fewer episodes of paroxysmal SVT. Lower plasma ionized calcium before dialysis was the only variable significantly associated with nonsustained VT, consistent with the literature data on hypocalcemia contributing to ventricular arrhythmias [24][25].
Echocardiographic data revealed that a lower LV ejection fraction <40% was associated with an increased incidence of AF paroxysms and more frequent ventricular arrhythmias. Our study demonstrated a positive relationship between an increase in LV mass index and AF. It should be noted that the results obtained are consistent with the literature data, in which lower cardiac output positively correlated with AF, nonsustained VT, and clinically significant arrhythmias in patients receiving dialysis [26]. These results need to be confirmed in other dialysis populations.
Conclusion
In patients receiving HD, older age, increased preload, and lower cardiac output are independently associated with clinically significant arrhythmias, and a positive association between increased LV mass index and AF episodes has been demonstrated. Lower cardiac output was positively correlated with AF and ventricular arrhythmias.
Relationships and Activities: none.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Likhachev-Mishchenko O.V., Kornienko A.A., Kornienko N.A., Kadyan E.G., Khaisheva L.A., Shlyk S.V. Prevalence of cardiac arrhythmias among patients undergoing chronic hemodialysis. Russian Journal of Cardiology. 2022;27(4S):4812. https://doi.org/10.15829/1560-4071-2022-4812
Copy