Clinical, laboratory and psychological aspects of moderate COVID-19 in cardiovascular patients one month after discharge from the hospital
In March 2020, the World Health Organization has declared the coronavirus disease 2019 (COVID19) outbreak a pandemic, which very quickly has covered the whole world [1]. Patients with such risk factors as hypertension (HTN), obesity, and diabetes were most often exposed to the virus, which makes this group of patients is of the greatest interest for observation. At the beginning of the pandemic, there was no data on the long-term COVID-19 outcomes. However, as the disease course was studied, more and more reports appeared in the literature about longterm damage not only to the lungs, but also to other organs and systems (heart, nervous system, kidneys, liver, and pancreas) [2]. There are following most commonly described symptoms: cough, shortness of breath, chest pain, weakness, fatigue, impaired sense of smell and taste, anxiety, sleep disturbance, and headaches. There are various definitions of the disease course as follows: acute COVID-19 lasting up to 4 weeks; ongoing symptomatic COVID-19 lasting up to 12 weeks; post-vaccination COVID19-like syndrome — a symptom complex that occurs within a few days after the introduction of vector vaccines resembling acute COVID-19. There have also been publications on so-called post-COVID-19 syndrome, which includes a wide range of systemic, cardiopulmonary, gastrointestinal, neurological and psychosocial symptoms [3][4]. There is no single definition of this concept today, just as there is no clear time frame for COVID-19 duration [3][5]. The authors describe symptoms of varying duration from 1,5 months to 1 year from the acute COVID19 onset [4-6]. There is not always a correlation between the severity of acute COVID-19 and related subsequent manifestations. So, if long-term convalescence in patients with severe COVID-19 looks natural, then prolonged symptoms in patients with milder disease forms, by analogy with other acute respiratory viral infections, are less explainable [7]. The etiology of all these abnormalities is not completely clear. According to some authors, the described manifestations may be the result of acute organ damage at the peak of illness; however, various clinical features of the infection still leave many questions [3]. That is why long-term follow-up of COVID-19 survivors is of great interest around the world.
The aim was to study clinical, laboratory and psychological aspects of moderate COVID-19 in cardiovascular patients one month after discharge from the hospital.
Material and methods
The first stage of the study was carried out during the work of the infectious diseases unit of the S.R. Mirotvortsev University Clinical Hospital № 1 (Saratov). The recruitment was carried out from November to December 2020. Patients were enrolled after condition stabilization, a few days before the expected date of hospital discharge. There were following inclusion criteria: age of 18-75 years, cardiovascular disease (ischemic cardiomyopathy, NYHA class I-III heart failure (HF), hypertension, stable angina, atrial fibrillation) and moderate COVID-19 confirmed by polymerase chain reaction test. In addition, the study included following exclusion criteria: non-consent to participate in research, inability to fill out informed consent and/or provided questionnaires on their own, patients of the intensive care unit with a severe COVID-19, NYHA class IV HF, grade III respiratory failure, severe dementia (Mini-Mental State Examination (MMSE) score of <10). During conversation with the patients, we collected past medical history, especially anamnesis of cardiovascular disease (CVD) and a detailed questioning about clinical performance of COVID-19. After the anamnesis collection, patients filled out few scales: the Hospital Anxiety and Depression Scale (HADS), the Beck Anxiety Inventory (BAI), and the Hamilton Depression Rating Scale. Data from physical examination, laboratory investigations (complete blood count, levels of transaminases, C-reactive protein, glucose, total cholesterol, low density lipoproteins), chest x-ray and computed tomography were evaluated. The severity of lung involvement was assessed according to the temporary guidelines of the Russian Ministry of Health (9th revision) [8].
One month after discharge from the infectious disease’s hospital, all patients were invited for examination and laboratory tests. Of the 88 patients, 72 respondents were in contact. There were no data on deceased patients. Patients were interviewed regarding the persisting clinical manifestations of COVID-19, their duration and length of the recovery period. All patients underwent blood sampling for complete blood count and biochemical tests. In addition, we performed electrocardiography, while HADS and BAI questionnaires were filled out.
For statistical processing, Excel (Microsoft Office 2016-2019 software package) and Statistica 8.0 software (Statsoft Inc., USA) were used. We analyzed the absolute number of events and their percentage. The cross-tabulation with ?2 test was used.
The study was carried out within the promising scientific research of the V.I. Razumovsky Saratov State Medical University (Russia) together with the Samarkand State Medical Institute (Uzbekistan) grant № INTI-SARGMU-SAMGMI-2021-03 “Prognosis of the development and personalized therapy of cardiovascular diseases in patients, including after COVID-19, based on clinical, laboratory, psychosocial factors and information technology”.
The study was performed in accordance with Good Clinical Practice and Declaration of Helsinki principles. The study protocol was approved by the Local Ethics Committee. Written informed consent was obtained from all participants.
Results
The study included 88 respondents, including 30 men and 58 women. The most numerous was the group of patients aged 41-75 years, regardless of sex. All patients who were in the hospital had moderate COVID-19 in accordance to the temporary guidelines of the Russian Ministry of Health (9th revision) [8].
As can be seen from Table 1, in CVD structure, the first place was occupied by HTN, coronary artery disease (CAD) and NYHA class 2 and 3 HF. The most common comorbidities were obesity, diabetes, nodular goiter, and lower limb varicose veins [4].
As standard therapy for CVD, 79,5% of respondents took angiotensin-converting enzyme inhibitors (sartans), 59,1% — beta-blockers, 47,7% — antiplatelet agents, 43,2% — statins, 36,4% — calcium channel blockers, 31,8% — diuretics, 4,5% — nitrates, amiodarone, alpha-blockers. For diabetes, 18,2% of patients took oral hypoglycemic agents, while 4,5% — insulin. In addition, 6,8% of the respondents took levothyroxine as hormone replacement therapy.
Some patients started treatment for COVID-19 even before admission to the hospital. Furthermore, 77,3% of respondents from the disease onset took antibiotics as follows: azithromycin, ceftriaxone and amoxiclav, both as monotherapy and in combination with each other; levofloxacin was prescribed much less frequently. Non-steroidal anti-inflammatory drugs were taken by 61,3% of patients, antiviral drugs — 38,6%, oral anticoagulants — 6,8%, while 6,8% of patients did not use any medications.
During hospitalization, symptoms of involvement of various organ systems were identified (Table 2). Both characteristic signs of COVID-19 (dry cough, shortness of breath, chest congestion, pain while breathing, loss of smell) and those less commonly mentioned in the literature: paresthesia, excessive sweating [9]. Attention was drawn to a large number of nervous system manifestations. Patients often complained of a feeling of fear and anxiety, disturbed sleeping, memory loss, dizziness, severe headaches that could not be relieved by analgesics and nonsteroidal anti-inflammatory drugs, lethargy, disorientation to place and time, and in some cases even hallucinations.
Among 97,7% of respondents with a fever, 54,5% had a temperature of 38,0-39,00C, while in 22,7% — >39,00C, in 20,5% — 37,0-38,00C. Oxygen insufflation required 47,7% of patients. The lowest saturation level was, on average, 88,9%.
In the hospital, blood pressure (BP) destabilization was often noted as follows: in 52,2% — hypertensive crisis, in 16,0% — episodes of hypotension. No patients with acute coronary syndrome and decompensated HF were identified.
After 1 month, among patients who complained of cough and shortness of breath during stay in the infectious disease’s hospital, the majority noted the disappearance or decrease in the severity of these symptoms. The complete relief of cough and shortness was noted by 52,8% and 8,3%, respectively. In addition, 50% of respondents noted a decrease in the dyspnea intensity, while only 5,5% observed an increase in its severity within a month after discharge. Complaints about a chest congestion and pain during breathing, registered in 13,6% of patients during hospitalization, were not detected in any of the respondents after discharge.
Attention is drawn to the long-term persistence of nervous system symptoms [10]. So, in 63,9% of patients who noted sleep problems during acute COVID-19, frequent night awakenings (19,4%), insomnia (16,6%), and long falling asleep (11,1%) remained. In addition, 55,5% mentioned memory loss. The feeling of fear and anxiety was recorded in 66,0% of and persisted in 36,0% of respondents for a month after discharge. It should be noted that taste and smell recovered completely or partially in 100% of the respondents, more often during the first 5-7 days after discharge. Such manifestations as lethargy, disorientation to place and time, headaches, dizziness, hallucinations, paresthesia, identified at the hospital stage, a month after discharge were not recorded.
Among other remaining manifestations, the leading positions are occupied by complaints of decreased exercise tolerance (80,5% out of 95,5% at the hospital stage), general weakness and excessive sweating (69,5%). Moreover, a slight decrease in exercise tolerance was noted by 38,9% of patients, while a moderate and pronounced decrease — by 30,5% and 11,1%, respectively. Slight, moderate and severe weakness was noted by 38,9%, 16,7% and 13,9%, respectively [11].
Further analysis revealed increased and decreased appetite in 22,2% and 16,7% of patients, respectively, compared to hospitalization period, and in 19,5% who complained of a complete loss of appetite during hospitalization, it restored to the previous level. Weight changes were noticed by 77,8% of patients. Of the 70,5% of respondents who lost weight during hospitalization, 52,8%, 19,5 and 5,5% recorded a slight (3-5 kg), moderate (5-7 kg) and significant (>10 kg) weight gain, respectively, while 8,3% did not notice a change.
It was also revealed that some of the organ system damage symptoms had not previously been registered (Table 3). Thus, 19,5% of the respondents for the first time in their lives shortness of breath during exercise, while 38,9% complained of hair loss (more often, women), 13,9% — fatigue, 16,7% — unsteady gait [12].
According to the survey, 64% of respondents noted a condition improvement within a month after discharge, while 33,3% — a slight improvement. In addition, 2,7% of respondents did not notice changes. A survey was also conducted about the identified adverse events and reasons for seeking medical attention. Within a month after discharge, 32 patients sought medical care, the most common reasons for which were gastrointestinal disorders — 25%, hypertensive crisis — 19,5%, stay in the rehabilitation unit after COVID-19 — 18,75%, progression of osteoarthritis — 12,5%, and decompensated diabetes — 6,25%.
As can be seen from Table 4, during the first month, BP destabilization was most often recorded in the form of episodes of BP increase and decrease during the day — 36%. Also, BP destabilization in the form of hypertensive crisis was also common — 14%. In 2,8% of patients, atrial fibrillation was registered for the first time, while 27,8% of the respondents did not note any adverse events.
Psychological assessment. During hospitalization, some patients had subclinical and clinical anxiety and depression according to the HADS [13].
One month after discharge, HADS revealed an increase in patients with subclinical and clinical anxiety 1 month after discharge compared with the period of hospitalization (Table 5). In addition, noteworthy is the increase in the level of depression among respondents. Thus, the level of clinical depression increased by more than 2 times 1 month after discharge from the infectious disease’s unit.
At the hospital stage, analysis of BAI data found that 97,7% of patients have a slight anxiety, and 2,3% — moderate. One month after discharge from the infectious disease’s hospital, these indicators did not change.
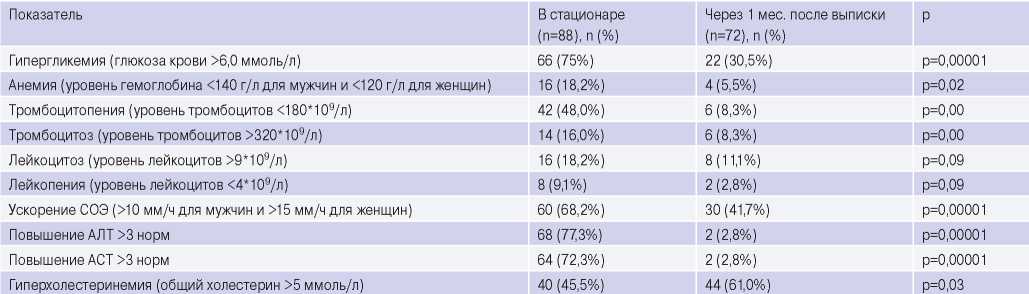
Analysis of laboratory tests. During hospitalization, blood tests revealed some features (Table 6). Hyperglycemia was detected in 75% of patients, both among individuals with and without prior diabetes. At the time of discharge, an increase in blood glucose levels was detected in 54,5% of them. Another feature is an increase in transaminases’ level 3 times above the reference values upon admission. An increase in alanine aminotransferase and aspartate aminotransferase levels was observed in 77,3% and 72,3%, respectively. At discharge, these indicators decreased to reference values in 95,5% of patients.
Complete blood count revealed erythrocyte sedimentation rate increase, which persisted at the discharge time in 65,9% of patients, leukocytosis, and mild anemia.
After 1 month, according to the complete blood count, there was a decrease in the number of patients with thrombocytosis, thrombocytopenia, anemia and leukopenia. However, in a number of patients, leukocytosis and leukopenia persisted.
Of the 30,5% of individuals with persistent hyperglycemia, according to a biochemical blood tests, only 27,3% have prior diabetes. Noteworthy is increase in a number of patients with hypercholesterolemia compared with inpatient period by 15,5% (6,8% of them with newly diagnosed hypercholesterolemia). Of the 25% of patients who were prescribed with lipid-lowering therapy, only 9,1% of the respondents complied with the recommendations within a month after hospitalization. An elevated transaminases’ level 3 times above the reference values persisted only in 2,8% of respondents and was associated with liver damage due to prior viral hepatitis.
Table 1
Characteristics of patients included in the study, % (n)

Note: * — there were no other types of atrial fibrillation in the study.
Abbreviations: HF — heart failure.
Table 2
Change in symptoms by organ systems 1 month after hospital discharge compared with inpatient period, n (%)

Table 3
New symptoms within 1 month after hospital discharge, n (%)

Table 4
Adverse events within a month after hospital discharge, n (%)

Table 5
Change in anxiety and depression levels 1 month after discharge, n (%)

Table 6
Changes in blood parameters 1 month after discharge, n (%)

Abbreviations: ALT — alanine aminotransferase, AST — aspartate aminotransferase, ESR — erythrocyte sedimentation rate.
Discussion
Undoubtedly, the presented study has a number of following limitations: small-scale, short-term, prospective design of the study, included patients with CVD and only moderate COVID-19. However, given the prevalence of CVD and the progression of COVID-19 pandemic, the study of this particular category of patients is necessary. This study focused on collecting anamnesis, symptoms and feelings of the patients themselves, in contrast to more numerous publications, which were based on the analysis of various paraclinical parameters.
The described cohort of patients is similar to those in other COVID-19 studies as follows: men and women are more often of the older age group, having CVDs (HTN, CHF, CAD), obesity, diabetes, and nodular goiter.
On the one hand, symptom changes in a number of patients in the form of a decrease in cough, shortness of breath, relief of chest pain while breathing is natural. On the other hand, long-term
persistence of shortness of breath in a large part of patients may have a combined character and indicate damage to both the respiratory and cardiovascular systems, and also have a central genesis. Particular attention is needed for patients who did not experience shortness of breath before COVID-19. This manifestation undoubtedly requires further study and clarification.
Attention is drawn to common nervous system damage and long-term persistence of various neurological symptoms (feelings of fear and anxiety, sleep disturbances, memory loss, lethargy, etc.). This can be compared with such a syndrome as “brain fog”, which includes symptoms, such as headache, confusion, impaired memory and attention. The first symptoms may appear within a few days from the disease onset and can persist for a long term. According to literature data, the manifestation of these symptoms may be associated with SARSCoV-2 presence in cerebrospinal fluid. It should also be noted that data appears in the literature that explain the clinical nervous system manifestations from a morphological point of view [14, 15]. The possible virus anterograde axonal transport along the olfactory nerve into central nervous system cells is described. There have been cases of detection and long-term virus persistence in neuronal and glial cells of the central nervous system [16]. All this may well explain the described clinical symptoms.
Identified mental abnormalities (increase in the number of respondents with subclinical and clinical anxiety and depression) on the one hand, of course, can be associated with emotional problems after the illness, but on the other hand, especially considering all of the above about nervous system damage, may indicate a long-term interaction of the virus (and/ or its consequences) with the nervous system. This also needs further study, long-term follow-up and may play a role in the further formation of concepts about the duration of COVID-19 itself and the postCOVID-19 syndrome [17][18].
Weight change, mainly weight gain up to 5 kg, is most likely natural, and may be a consequence of appetite restoration after its decrease during hospitalization.
Prolonged weakness, lasting at least a month in a significant number of patients, is also likely a COVID-19 hallmark. Many patients note a decrease in exercise tolerance, persistence of severe, moderate, or slight weakness after recovery. Accumulated evidence suggests that angiotensin converting enzyme 2 receptors are in skeletal muscle [19]. When the virus enters the body, it interacts with these receptors, followed by damage to muscle mitochondria, which leads to muscle weakness.
Hair loss, observed in a significant number of respondents, has no reliable etiological explanation and requires further study. The most popular theory regards telogen effluvium, which describes abnormal shift in follicular cycling and their loss under the influence of adverse factors, including severe diseases [20]. Since we studied a homogeneous sample with only moderate COVID-19, we cannot confirm this pattern.
Laboratory alterations can be explained as follows: the observed decrease in the number of patients with leukocytosis and increased erythrocyte sedimentation rate may indicate an expected inflammation reduction. However, the increase in the number of patients with hypercholesterolemia draws attention. Perhaps this is due to atherosclerosis activation against the background of an infectious disease, possibly with low adherence to lipid-lowering therapy. However, a direct relationship with the SARS-CoV-2 should be ruled out in further studies.
The abundance and various duration of manifestations indicate the need for long-term follow-up of patients, both within the further research and in routine practice. A clear understanding of symptom dynamics in patients after COVID-19 plays an important role in differential diagnosis with other diseases and in the choice of patient management tactics.
Conclusion
Within 1 month after hospitalization, a regular decrease in the number of patients with respiratory symptoms is observed. There is a decrease in the number of patients with complaints of cough and shortness of breath, as well as the disappearance of symptoms such as chest congestion and pain when breathing. However, long-term damage to other organs and systems persists. The most significant symptoms are BP destabilization, tachycardia, shortness of breath, a sense of fear and anxiety, lethargy, and excessive sweating. The following symptoms appear: shortness of breath during exercise without such disorders before the disease, fatigue, unsteady gait, and hair loss. There is an increase in the number of patients with subclinical and clinical depression according to the HADS scale by more than 2 times. Among the laboratory parameters, an increase in the number of patients with increased total cholesterol and low-density lipoproteins was revealed.
Relationships and Activities. The study was carried out within promising research of the V.I. Razumovsky Saratov State Medical University in cooperation with the Samarkand State Medical Institute (Republic of Uzbekistan) № INTI SARGMUSAMGMI-2021-03 “Prediction of the development and personalized therapy of cardiovascular diseases in patients, including those after COVID-19, based on clinical, laboratory, and psychosocial factors and information technologies”.
Чтобы читать статью войдите с логином и паролем от scardio.ru
Keywords
For citation
Tyapaeva A.R., Semenova O.N., Tashkenbaeva E.N., Nasyrova Z.A., Naumova E.A. Clinical, laboratory and psychological aspects of moderate COVID-19 in cardiovascular patients one month after discharge from the hospital. Russian Journal of Cardiology. 2021;26(4S):4603. https://doi.org/10.15829/1560-4071-2021-4603
Copy